
NewsRoom
LabNotes - September 2012
Transforming Energy Technologies with Nanomaterials
Nanotechnology is the manipulation of matter on an atomic and molecular scale, generally with materials and devices sized from 1 to 100 nanometers (about 1/75,000ths the width of a human hair). At this scale, quantum mechanical and other size-based effects strongly influence a material's physical and chemical behavior in ways not predictable from bulk properties in the macroscopic realm. National Energy Technology Laboratory (NETL) researchers are exploiting these effects to transform a wide range of nanomaterials into viable energy technologies.
Visible Light Photochemical Conversion of Carbon Dioxide with Heterostructured Titania
Managing CO2 emissions is one of the largest political and technical challenges currently associated with the use of fossil fuels, so a variety of CO2-eliminating or reducing technologies are now being investigated. One promising approach involves the light-mediated (photo-catalytic) reduction of CO2 into useful chemicals such as methane and methanol. These chemicals can be used directly as fuels, sold to offset carbon capture and storage costs, or transformed into a variety of other useful chemical products.
 |
| Forming heterostructures of lead sulfide with titanium dioxide creates a highly active catalyst capable of utilizing visible light for photoreducing carbon dioxide. (Alt text: Illustration of new catalyst able to use visible light to photoreduce CO2.) |
In addition to being a pigment for white paint, titania, or titanium dioxide, is a popular material for converting carbon dioxide with sunlight because it is abundant, inexpensive, and demonstrates reasonable catalytic activity. Despite these attributes, the efficiency of titania for photovoltaic (solar cell) applications and light-facilitated catalytic applications is severely limited by the energy required (band gap) to activate the titania. Titania is normally only active in the presence of ultraviolet (higher energy) light meaning it only utilizes 4-5% of the solar energy reaching the earth's surface. In an ideal case, titania would also be capable of harnessing lower-energy visible and near-infrared light in order to better use more of the sun's output for these applications. Historical approaches for improving titania's ability to harness the low energy tails of the solar spectrum involve the use of dye sensitizer molecules or the introduction of impurities such as nitrogen or sulfur. While these approaches have shown promise for increasing titania's photoactivity, there is still room for transformational advances in efficiency.
One approach for improving titania's use of the solar spectrum involves decorating the catalyst's surface with semiconductor quantum dots (QDs), such as cadmium selenide or lead sulfide, to form a heterostructured material. The QDs in the heterostructure absorb light strongly and this activity can be systematically tuned from the green to near infrared edges of the solar spectrum by controlling the size and composition of particle employed. Using this approach, NETL investigators were the first to demonstrate the visible-light initiated photoreduction of CO2 with a technically viable catalyst system. These heterostructures utilize almost 40% of light reaching the earth's surface and have reactivities that are up to 4 times faster than plain titania. Based on energetic considerations, several other semiconductor QDs should also be equally efficient at sensitizing titantia for CO2 photoreduction. Current research in this area is addressing the dynamics of electron transfer across the QD-titania interface and investigating the ability of plasmonic heterostructures to enhance the catalytic reactivity of titania and other metal oxides.
Unprecedented Electrocatalytic CO2 Conversion Efficiency with Au25 Nanoclusters
In addition to the photochemical transformation of CO2, NETL researchers have been investigating electrocatalytic conversion approaches using a new class of ligand-protected gold clusters as catalysts. These clusters are composed of exactly 25 gold atoms (Au25) with associated capping ligands. These sub-2nm sized clusters span the scale between molecules and traditional nanoparticles making them an interesting platform for exploiting properties unique to both size regimes. As such, Au25 has a variety of unique properties including a ground state anionic (negative) charge and molecule-like electronic states.
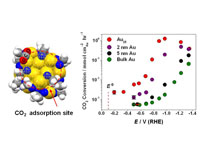 |
Nanoclusters containing precisely 25 gold atoms show unprecedented efficiency for the electrocatalytic conversion of carbon dioxide to carbon monoxide.
(Alt text: Illustration of nanoclusters containing 25 gold atoms electrocatalytically convert carbon dioxide to carbon monoxide.) |
Gold traditionally enjoys a reputation as an inert metal and CO2 shows little electronic or chemical interaction with traditional gold surfaces. However, NETL's computational efforts suggested—and later experiments confirmed—that electrostatic interactions between CO2 and the negatively charged Au25 cluster perturb its electronic structure and impact optical properties. Experimental results with Au25 clusters indicate a reversible Au25−CO2 interaction that produced spectroscopic changes similar to those seen with cluster oxidation. The observation of an unexpected spontaneous coupling between the negatively charged Au25 cluster and CO2 suggested Au25 could also catalyze the reduction of CO2, motivating NETL researchers to further investigate this application.
Typical electrocatalysts require large voltages to overcome kinetic limitations and convert CO2 into useful products, ultimately creating a challenge for large-scale deployment. In this application, Au25 catalyzed the two-electron reduction of CO2 into carbon monoxide (CO) at voltages close to the thermodynamic limit. This unprecedented efficiency far exceeds that of larger, more-traditional, gold nanoparticles, as well as macroscopic, bulk, gold foils. Moreover, Au25 showed a peak CO2 to CO conversion that was nearly 100 percent efficient and proceeded at rates 7−700 times higher than those found in tests with gold catalysts having larger particle sizes. The catalytic rates noted for CO2 to CO conversion with Au25 were 10−100 times higher than those reported in the literature for other state-of-the-art electrocatalytic technologies.
Current research at NETL is focusing on how to efficiently scale this process and whether or not similarly sized clusters of other catalytic metals, such as copper and nickel, can also be utilized.
Tuning the Reactivity of Copper Nanoparticles with Organic Capping Ligands
Organic ligands are commonly used as stabilizing agents during the synthesis of nearly all nanomaterials, but little is known about their effect on catalytic reactivity. Metal oxide nanoparticles are an interesting platform for addressing ligand effects because the surface structure, composition, and oxidation state of the oxide can dramatically influence catalytic activity. To address these issues, NETL researchers have used a variety of copper oxide nanoparticles to evaluate how the organic capping ligands decorating the surface of these catalysts impact performance. The electroreduction of CO2 and electroxidation of methanol were used as model reactions for probing activity because of their applications in CO2 management and fuel cell applications, respectively.
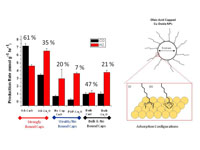 |
The strong chemical binding of oleic acid capping molecules to the surface of copper oxides stabilizes reactive sites and improves their electrocatalytic reactivity for converting carbon dioxide to carbon monoxide.
(Alt text: Illustration of catalyst whose reaction converts carbon dioxide to carbon monoxide.) |
Oleic-acid capping ligands were found to bind strongly to the surfaces of both copper (I) oxide and copper (II) oxide (CuO and Cu2O) nanoparticles (see figure). In situ Raman spectroscopy during CO2 electroreduction to CO discovered that the oleic-acid caps stabilize both the CuO and Cu2O oxides preventing the catalyst from being reduced to metallic copper during the reaction. The oleic acid ligand's ability to prevent the reduction of the copper oxide also has a dramatic impact on its performance as CO2 reduction catalyst. The oleic acid capped nanoparticles were 3-5 times more selective for the production of CO over H2 and nearly 7 times more reactive than copper nanoparticles with weakly bound or no capping ligands.
The oleic acid ligand's role on methanol oxidation was also linked to its ability to stabilize the copper oxidation state during the reaction. In this reaction, the oleic acid capped materials are nearly 100% selective for the production of formaldehyde over CO2 creating this chemical 10-100 times faster than copper oxides with weakly bound or no organic capping ligands. The NETL results were the first to directly demonstrate the role of capping ligands on reactivity and show how the performance of catalysts for energy-related applications could be tuned through surface functionalization.
Watching Individual Atoms React on Iron-based Hydrogen Production Nanocatalysts
While it has been known for some time that certain iron species can impact the water gas shift reaction (WGS) for producing hydrogen from fossil based resources, the precise nature of these species is poorly understood. Being able to directly see how water, carbon monoxide, carbon dioxide and hydrogen interact with a catalyst surface would enable scientists and engineers to design better performing catalysts.
NETL researchers have been working on a variety of iron- and iron oxide-based nanocatalysts to develop a better understanding of how these materials perform in real-world applications. A key strategy to their approach is to first grow nanoscale iron species on atomically flat substrates. These nanomaterials are the most accurate reproduction of the size, structure, defects, and reactive sites present in real-world catalysts. The scientists then used specialized in situ analytical methods that allow them to evaluate reactions on these nanocatalysts at conditions more closely representing the applications they are used in. In this regard, the team utilizes the state-of-the-art Ambient Pressure X-ray Photoelectron Spectroscopy (AP-XPS) instrument at Lawrence Berkeley National Laboratory in conjunction with an in situ scanning tunneling microscope (STM) housed at NETL. The combination of these two techniques allows them to identify the reactions occurring on these nanocatalysts and directly image the atoms involved.
 |
The atoms involved in the dissociation of water on iron oxide nanoparticles can be directly visualized with NETL's scanning tunneling microscope. The white dots forming around the periphery of the triangular particle are hydroxl (OH) functional groups formed from the dissociation of water at the particle's edges.
(Alt text: Scanning tunneling microscope images of iron oxide nanoparticles.) |
The NETL team found that the dissociation of water occurs on Fe-oxide nanoparticles at pressures far below ambient moisture conditions. The reaction results in the production of adsorbed hydroxyl groups (OH) forming on the Fe-oxide particles and was observed using AP-XPS from low to relatively high water pressures. The team was also able to successfully image individual hydroxyl groups forming around the periphery of the Fe-oxide particles in real time using in situ STM (see image). The hydroxyl groups decorate the outermost edge of the Fe-oxide particles with the liberated oxygen atom becoming directly incorporated into the oxide crystalline lattice. After the edge of the particle becomes completely decorated with hydroxyl groups, the reaction stops and the hydroxyl groups are stable in these reaction sites up to several hundred degrees centigrade.
This work illustrates that the structural defects and under-coordinated sites existing at the edges of Fe-oxide catalysts are the active chemical centers directing the performance of the catalyst. It also points to the role that H2O has on the deactivation and passivation of the catalysts. Learning to intentionally design catalysts with specific defects will open the door to a new approach for improving catalyst performance.
|