- Home
- Slides
- Home
- Tools and Resources
- Research Summaries for Consumers, Clinicians, and Policymakers
- Search for Research Summaries, Reviews, and Reports
- Research Available for Comment
- Submit a Suggestion for Research
- Submit Scientific Information Packets
- Comparative Effectiveness Research Grant and ARRA Awards
- News and Announcements
- What Is Comparative Effectiveness Research
- Who Is Involved in the Effective Health Care Program
- What Is the Effective Health Care Program
Slides
Slides: 109–120 of 624
Evidence of rFVIIa Use for Bleeding Secondary to Brain Trauma vs. Usual Care
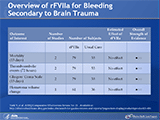
Overview of rFVIIa for Bleeding Secondary to Brain Trauma
Evidence of rFVIIa Use for Adult Cardiac Surgery vs. Usual Care
Overview of rFVIIa for Adult Cardiac Surgery: Clinical Outcomes
Increased Risk of Thromboembolic Events With rFVIIa Use for Adult Cardiac Surgery
Evidence of rFVIIa Use for Pediatric Cardiac Surgery, Liver Transplantation, and Prostatectomy vs. Usual Care
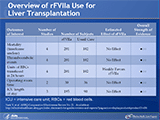
Overview of rFVIIa Use for Liver Transplantation
Overview of rFVIIa Use for Pediatric Cardiac Surgery
Overview of rFVIIa Use for Prostatectomy
Summary of Outcomes for Most Common Off-Label, In-Hospital Uses of rFVIIa
Additional Off-Label Uses of rFVIIa Requiring Future Research
Additional Off-Label Uses of rFVIIa Requiring Future Research
Your slide tray is being processed.


 E-mail Updates
E-mail Updates