- News
- Overview
- Research
- Multimedia
- Related Links
News
 |
Mercury is a toxic pollutant. In its elemental form, gaseous mercury has a long residence time in the atmosphere, up to a year, allowing it to be transported long distances from emission sources. Mercury can be emitted from natural sources such as... |
Tuesday, November 13, 2012 Type: Publication
|
 |
Electron beam microanalysis of coal samples in U.S. Geological Survey (USGS) labs confirms that As is the most abundant minor constituent in Fe disulfides in coal and that Se, Ni, and other minor constituents are present less commonly and at lower concentrations than those for As. |
Tuesday, May 01, 2012 Type: Outside Publication
|
 |
Dozens of natural and human-induced coal fires are active in the Powder River Basin (PRB) of southeast Montana and northeast Wyoming, the largest source of coal produced in the United States. Coal fires exhibit surface features similar to those found above high-temperature geothermal systems... |
Tuesday, March 06, 2012 Type: Outside Publication
|
 |
The principal mission of the USGS Energy Resources Program is to (1) understand the processes critical to the formation, accumulation, occurrence, and alteration of geologically based energy resources; (2) conduct scientifically robust assessments of those resources; and (3) study the impacts of... |
Sunday, December 04, 2011 Type: Publication
|
 |
Geochemistry is a constantly expanding science. More and more, scientists are employing geochemical tools to help answer questions about the Earth and earth system processes. Scientists may assume that the responsibility of examining and assessing the quality of the geochemical data they generate is... |
Tuesday, September 13, 2011 Type: Publication
|
 |
Coalbed methane (CBM) is a source of natural gas that is recovered by dewatering coal beds and collecting volatile compounds, such as methane, that are desorbed from the coal. Coalbed methane currently accounts for approximately 7% of U.S. natural-gas production (U.S. Energy Information... |
Monday, September 12, 2011 Type: Outside Publication
|
 |
It is with great pleasure that we invite you to the 10th International Conference on Mercury as a Global Pollutant (ICMGP) to be held in Halifax, Nova Scotia, Canada from July 24-29, 2011. |
Friday, January 28, 2011 Type: Conference
|
 |
Constraints on drainage management in the western San Joaquin Valley and implications of proposed approaches to management were recently evaluated by the U.S. Geological Survey (USGS). |
Monday, September 27, 2010 Type: Publication
|
 |
This study presents >5 cumulative years of tropospheric mercury (Hg) speciation measurements, over the period of 2003–2009, for eight sites in the central and eastern United States and one site in coastal Puerto Rico. |
Wednesday, September 22, 2010 Type: Outside Publication
|
 |
Atmospheric emissions, transport and deposition of mercury (Hg) are key processes leading to the global mercury contamination problem (Wiener et al., 2003). Mercury emissions to the atmosphere arise from natural and anthropogenic sources, and by re-emission of previously deposited... |
Monday, May 17, 2010 Type: Outside Publication
|
Overview

Figure 1. Simplified biogeochemical cycle of
Mercury in the environment:
View larger image. 
Photo: Blebs of elemental mercury exposed in a
breached conduit at the defunct Nikitovka
mercury plant, Gorlovka, Ukraine.
Mercury (Hg) is an important trace element present in the environment because 1) mercury can occur in organic forms, such as methylmercury, which are highly toxic and can be bioconcentrated, biomagnified, and bioaccumulated in the food web, and 2) as an atmospheric constituent mercury can be transported long distances from its original source and eventually deposited back to the surface, possibly hundreds or thousands of miles away.
Mercury has a complex biogeochemical cycle which allows it to be transferred between different ecosystem reservoirs and exhibit chemical transformations that control its behavior and toxicity (Figure 1.). Researchers in the USGS Eastern Energy Resources Science Center and their colleagues are actively involved in studying the behavior and occurrence of mercury in the environment, as well as human exposures to mercury. Currently research is focused in five areas.
Allan Kolker
Project Chief
Mark Engle
Lead Scientist
Selenium is a naturally occurring element and an important micronutrient essential for all living organisms. Selenium is also used in many commercial applications including glass decolorizing, metallurgical additives, machining of ferrous and nonferrous alloys, pigments, copier photoreceptors, and in semiconductor and photocell industries.
Although selenium is essential, large concentrations can be toxic, particularly to aquatic wildlife. Human activities such as coal mining, industrial processing, agricultural runoff and leaching of natural soluble selenium can concentrate selenium in excess of the regulatory limits of 5 micrograms per liter (µg/L) and affect wetland habits. The USGS Energy Resources Program researches the geologic location, extent and distribution, and environmental affects on human health and the landscape and provides this information to decision-makers.
Frequently Asked Questions Pertaining to Selenium
Decision Analysis
Frank Dulong
Lead Scientist
Top of Page
Research
Mercury Emissions from China
Mercury Emissions from China
The Peoples' Republic of China produces and consumes the largest quantity of coal in the world; about 1.7 billion tons produced in 2003 (Figure 1). Current projections, rough estimates at best, forecast coal consumption to be 3.3 billion tons by 2020. Although the nationwide percentage of electrical production from coal is falling due to increased power generation efficiency, China will burn more coal than any other country for the... [+]
foreseeable future. China also is estimated to be the largest producer of mercury emissions (Dastoor and Larocque, 2004). A recent comprehensive study of anthropogenic mercury emissions in China (Streets et al., 2005) yielded a figure of 536 t of mercury for the year 1999 with coal combustion (all types) accounting for 38% of the total. Atmospheric mercury emission is an international problem as the troposphere provides effective global transport of mercury. Although the estimates vary, China produces about three times more mercury per ton of coal burnt than the USA because of the lack of modern pollution technology and limited use of cleaned coal. Knowledge of the mercury content, mode of occurrence, and regional distribution in Chinese coal is vital in order to assess the global atmospheric contribution from Chinese coal combustion.
Under the aegis of the USGS WoCQI Program, we have collected and analyzed 305 Chinese coal samples from mines with the highest production from 25 provinces, municipalities, or autonomous regions, which thus reflects much of the coal currently supplied for power generation and industrial use. The method of determination was routine cold-vapor atomic absorption spectroscopy using two methods of sample dissolution. The arithmetic mean, on an as-determined, whole-coal basis, of 305 samples is 0.15 ppm (1 sigma = 0.14), with a minimum of 0.02 ppm and a maximum of 0.69 ppm. All data averaged to a dry basis is 0.16 ppm. A small percentage (~ 9 %) of the mercury data were below the method detection limit of 0.02 ppm. Duplicate analyses and inter-laboratory comparisons suggest that analytical uncertainties can arise from imperfect sample splitting using a mesh sample size in some samples.
Research continues to assess relationships between mercury content in coal and other measured chemical and physical parameters and topical studies related to mercury mineralization in coals of Guizhou Province.
References
Dastoor, A.P., Larocque, Y., 2004, Global circulation of atmospheric mercury: a modeling study: Atmospheric Environment, v. 38, p. 147-161.
Streets, D.G., Hao, J., Wu, Y., Jiang, J., Chan, M., Tian, H., Feng, X., 2005, Anthropogenic mercury emissions in China, v. 40, p. 7789-7806.
Mercury Deposition Network
Mercury in Coal
USGS research provides information on the amount and forms of mercury and other elements present in U.S. Coals (Tewalt et al., 2001). Understanding the distribution of mercury in coal is especially important in light of recent U.S. EPA rulings, outlining plans to limit mercury emissions from U.S. coal-fired utilities. Using selective chemical leaching (Figure 1), laser-ablation ICP-MS (Figure 2), and other approaches, USGS results... [+]
show that pyrite is the primary host of mercury in bituminous coals, whereas the proportion of mercury present in organic parts of coal is generally greater in low-rank (lignite and sub-bituminous) coal. Many Eastern U.S. bituminous coals are “cleaned” prior to use in utility power stations, to reduce sulfur emissions. This coal preparation reduces sulfur content, primarily by removing pyrite from coal. In doing so, a portion of the mercury present may also be removed, as a co-benefit to sulfur reduction. Currently about 35% of the mercury that goes into coal-fired utility power stations in the U.S. is captured by air pollution control devices, whose primary function is to trap particulates from coal combustion, or sulfur emitted from flue gasses. Under the EPA Clean Air Interstate Rule (CAIR), the initial phase of mercury reductions from coal-fired utility power station will primarily result as a co-benefit of reducing other pollutants with existing control technology. Under the second phase of the plan, the EPA Clean Air Mercury Rule (CAMR), greater reductions of mercury emissions are specified, probably requiring new mercury-specific control technology for utilities. By understanding the mode of occurrence of mercury in coal, and the concentration of constituents that affect capture of mercury, such as chlorine, the USGS provides information needed to help predict and control mercury emissions.

Figure 1. Selective leaching results for 15 coal samples (12 from the U.S.). Yellow bars indicate the proportion of mercury leached with nitric acid, thought to be contained in sulfide minerals, such as pyrite. The fraction of mercury not leached (100 percent minus total bar height) represents organic-associated mercury not removed by the leaching process. Arrows indicate minimum values. Figure is from Palmer and others, 1998.

Figure 2. Plot of mercury in pyrite plotted versus arsenic content (log scale) based on reconnaissance laser ablation ICP-MS analysis of 6 U.S. eastern bituminous coal samples. Data points represent individual analysis points ranging in size from 10 to 50 micrometers. Results for pyrite grains analyzed indicate mercury concentrations that are 10’s to 100’s of times higher than mercury concentrations in the whole coal. Samples include an Ohio 5/6/7 blend (Ohio), Illinois #6 (Illinois), Pittsburgh Coal from West Virginia (Pitts), the Warrior Basin of Alabama (AL), the Upper Coalburg Coal from West Virginia (WV), and an eastern Kentucky coal (KY). Data are from Diehl and others, 2005 and Kolker and others, 2002.
References
Diehl, S.F., Goldhaber, M.B., Tuttle, M.L.W., Ruppert, L.F., Hatch, J.R., Koenig, A.E., and Lowers, H.A., 2005, Distribution of arsenic, selenium, and other trace elements in pyrite-filled structures in Appalachian coals of Alabama, Kentucky, and West Virginia: Proceedings of the 22nd International Pittsburgh Coal Conference, Pittsburgh, PA, September, 2005, 24 p., CD-ROM.
Kolker, Allan, Mroczkowski, S. J., Palmer, C. A., Dennen, K. O., Finkelman, R. B., and Bullock, Jr., J. H., 2002, Toxic substances from coal combustion- A comprehensive assessment, Phase II: Element modes of occurrence for the Ohio 5/6/7, Wyodak, and North Dakota coal samples: U.S. Geological Survey, Open File Report 02-224, 79 p.
Palmer, C. A., Mroczkowski, S. J., Finkelman, R. B., and Crowley, S. S., 1998, The use of sequential leaching to quantify the modes of occurrence of elements in coals: Proceedings of the Fifteenth Annual International Pittsburgh Coal Conference, 28 p., CD-ROM.
Mercury Deposition Network
Mercury Deposition Network
The USGS in cooperation with researchers at the George Mason University Department of Chemistry, operate a mercury deposition precipitation sampler in Culpeper, Virginia. The site is part of the Mercury Deposition Network (MDN), a cooperative U.S. and Canadian network of mercury wet deposition samplers deployed to characterize mercury deposition via rain and snow (i.e., wet deposition). The sampler has been running... [+]
continuously since November of 2002 and was modified to collect samples for trace metal analysis beginning in November of 2004 (Figure 1).

Figure 1. View of the VA-08 MDN site showing the recording rain gauge (foreground) and an MDN precipitation collector (background). Precipitation samples are collected through one of two chimneys on the left side of the collector (one for mercury and one for other trace metals) when the moisture sensor activates the lid covering the sample chimneys. Photo by Allan Kolker, USGS.
Data generated from the sampler include weekly mercury and trace element concentration and deposition. These data show variations in mercury and other trace metal wet deposition across the country (Figure 2). Statistical and air mass trajectory modeling are being used to identify potential sources for the mercury and other trace metals, and suggest processes important in controlling wet deposition. Data from the VA-08 site are also being compared to data from the nearby Shenandoah National Park-Big Meadows MDN site (VA-28). The sites are within 31 kilometers of one another but vary in elevation (VA-08 = 183 m; VA-28 = 1,074 m) allowing for investigation on the effect of elevation on deposition.

Figure 2. MDN results for 2004, including the Culpeper VA-08 site. Warmer colors indicate areas of higher wet deposition while cooler colors represent lower yearly deposition. Mercury deposition and concentration maps available from the National Atmopsheric Deposition Program – Mercury Deposition Network.
Atmospheric Mercury Speciation
Atmospheric Mercury Speciation
The behavior of mercury in the atmosphere depends upon its form, or species. Elemental mercury (Hgo) is typically not very reactive with a global lifetime of a few months to a year and is thought to be transported significantly in the troposphere. Reactive gaseous mercury (RGM) species, are not well characterized chemically but are thought to be gaseous Hg(II)-bearing molecules such as HgCl2(g). RGM species are... [+]
notable for being quickly deposited from the atmosphere to the surface and are thought to be readily available for conversion to methyl mercury, a highly toxic form of mercury. Particulate mercury (Hg-P) is also quickly deposited and is often found in high concentrations near combustion sources. Although much lower in proportion than Hgo, the greater reactivity and deposition rates of RGM and Hg-P make them a greater environmental concern. Chemical reactions that occur in the atmosphere can transform mercury between these various species.

Figure 1. USGS Mobile Mercury Laboratory operated by the USGS Wisconsin Water Science Center. Instruments include an atmospheric mercury speciation unit; NOx, SOx, O3, and PM2.5 analyzers; a meteorological station; and a wet deposition sampler. Photo by James Robinson, USGS.
Recently, USGS researchers from the Energy Resources Program and the Water Mission Area have started characterizing tropospheric Hgo, RGM, and Hg-P concentrations in southern Alabama, near the Gulf of Mexico. The project relies upon the USGS Mobile mercury laboratory (Figure 1) which measures near real-time concentrations of the three mercury species (Figure 2). Potential sources of mercury to the region include a nearby coal-fired power plant and industrial emissions from nearby cities such as Mobile, Alabama; Pensacola, Florida; and New Orleans, Louisiana. Natural processes are also being investigated that could cause conversion of Hgo to RGM or Hg-P.

Figure 2. Plot of preliminary RGM, Hg-P, and Hgo concentration data determined by the atmospheric mercury speciation unit on the USGS Mobile Mercury Laboratory deployed at Weeks Bay, Alabama from 6/6/05 to 6/16/05 (Julian days 157 to 167). The gap in the data is a result a shut down during tropical storm Arlene.
Additional total suspended particulate samplers were used to collect atmospheric particulates on filters that are analyzed for mercury as well as a variety of other constituents (Figure 3). Combining the mercury speciation data with multivariate statistical methods and air mass trajectory modeling, potential sources of mercury and other trace metals can potentially be identified as well as processes that produce RGM and Hg-P.

Figure 3. High-volume (left) and low-volume (right) total suspended particulate samplers deployed at Gulf Shores State Park, Alabama. The high volume sampler collects particles on a large filter located under the triangular lid. The high volume filters were analyzed for mercury and soluble components including Cl and SO4. The low volume sampler contains two filters housed in filters packs at each end of the tripod crossbar. One filter is analyzed for mercury and the other for other trace metals. Photo by Allan Kolker, USGS.
Mercury Exposure in Ukraine
Mercury Exposure in Ukraine
High levels of mercury are present in Gorlovka, Ukraine (pop. 320,000), due to mining and processing of mercury that ceased in 1991, leaving a series of abandoned open pits, a now closed mercury extraction plant (Figure 1), extensive mine tailings, and several tailings ponds. Past use of extreme mercury-enriched coal (Figure 2), produced as a byproduct of mercury mining, and current industrial use of local coal from nearby mines, pose... [+]
potential hazards to Gorlovka. An integrated environmental/human health study is underway to define mercury exposure levels, assess possible health effects to exposed individuals, and determine the feasibility of further epidemiologic studies on a larger scale. The study, Feasibility of Assessing Health Risks from Long-term Mercury Exposure in Gorlovka, Ukraine funded by the U.S. Civilian Research and Development Foundation, is part of USGS studies on health effects of inorganic substances in coal.

Figure 1. Defunct mercury processing plant at Nikitovka Mines, Gorlovka, Ukraine, operated from 1968 to 1991. A small portion of this site is currently used to recycle fluorescent lamps and batteries to recover mercury. Photo by Allan Kolker, USGS.
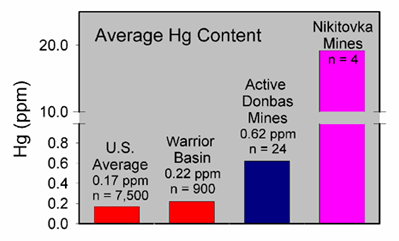
Figure 2. Average mercury content of four Nikitovka coal samples collected by USGS scientists and collaborators at Donetsk National Technical University, compared to mercury in active mines of the region and U.S. averages for in-ground coal, and for the Warrior Basin of northern Alabama. U.S. coal data are from Bragg et al., 1998. Note break in vertical scale between 1 and 10 ppm mercury.
Current work on human mercury exposure in Gorlovka began in August, 2005, following a more general study of mercury-rich coals in the region, funded by the NATO Science Program (Figure 2). In the current study, a group of 30 workers at a mercury recycling facility on the site of the defunct mercury extraction plant was selected for sampling hair, nails, blood, and urine, to assess their mercury exposure. In conjunction with tissue sampling, environmental samples were taken to assess mercury levels and potential exposure near the mercury mines and over a larger portion of Gorlovka (Figure 3). Additional tissue sampling is planned for non-exposed individuals in Gorlovka and in a nearby control municipality. Our work in Ukraine has the potential to be an important case study of human exposure to mercury.

Figure 3. Environmental sampling in Gorlovka by Kathryn Conko (USGS) to assess exposure of residents to mercury. Photo by Allan Kolker, USGS.
Top of Page
Page Last Modified: Friday, October 05, 2012
|
|
Mercury and Selenium Topics
Publication Search
A searchable database of thousands of published sources, dating back several decades
Find Data
USGS Energy Data Finder: Download GIS and tabular data, databases, geospatial web services (ArcGIS, WMS, KML)
EnergyVision
A single map viewer portal incorporating a range of maps, data and services
|