By: Carol Holquist
It’s that time of year again – cold and flu season. For parents of infants and children, it can sometimes be difficult choosing the right medicine from the pharmacy, especially when it contains acetaminophen. And once you have made your choice, sometimes the correct dosing for your infant or child can be a challenge!
I wanted to let you know that the FDA has issued a Drug Safety Communication aimed at giving consumers like you more information about how to avoid confusion and potential dosing errors when you are giving your infant products containing acetaminophen.
There are many different brands of acetaminophen marketed for “infants” to choose from in the store. We recommend that you carefully read the labels of liquid acetaminophen before giving the medication to your child. Giving more than the recommended dose of acetaminophen can cause serious side effects and even death.
Recently, manufacturers of acetaminophen made changes to the type of concentration available for use. Until now, liquid acetaminophen marketed for “infants” has only been available in a stronger concentration that doesn’t require giving infants as much liquid with each dose. Now there is a less concentrated form of liquid acetaminophen marketed for “infants” and both concentrations of liquid acetaminophen are in circulation.
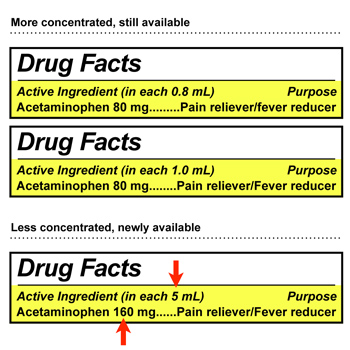
It can be difficult to tell the two different concentrations marketed for “infants” apart from one another because all of the product labels look very similar. We are concerned that infants and children could be given too much or too little of the medicine if the different concentrations of acetaminophen are confused. Before giving the medication, parents and caregivers need to know whether they have the less concentrated version or the older, more concentrated medication.
Here are some things you can do:
Please consult your pediatrician before giving this medication to make sure you’re both talking about the same concentration.
Read the Drug Facts label on the package very carefully to identify the concentration of the liquid acetaminophen, the correct dosage, and the directions for use:
- Look for the “Active ingredient” section of the Drug Facts usually printed on the back of an OTC medication boxes.
- If the package says “160 mg per 5 mL” or “160 mg (in each 5 mL)”, then this is the less concentrated “infant” acetaminophen. This medication should come with an oral syringe to help you measure the dose.
- If the package says “80 mg per 0.8 mL” or “80 mg per 1 mL,” then this is the more concentrated “infant” acetaminophen. This product may come with a dropper.
- Use only the dosing device provided with the purchased product.
- Consult your pediatrician before giving this medication and make sure you’re both talking about the same concentration.
If the dosing instructions provided by your healthcare provider differ from what is on the product’s label, then check with a healthcare professional before giving the medication. If your child is under two years of age there will be no dosing provided on the products marketed for “infants”. Please contact your healthcare provider for dosing information for children under two years of age and remember to tell them which type of medicine you have. Do not rely on dosing information provided from other sources such as the Internet, old dosing charts, or family members.
Our goal is to protect the public health of all Americans, and we hope that this additional information on dosing for your child will be helpful.
Carol Holquist is director of FDA’s Division of Medication Error Prevention and Analysis.


