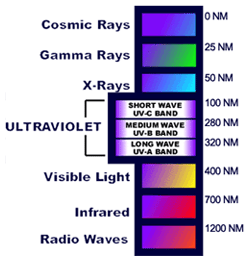
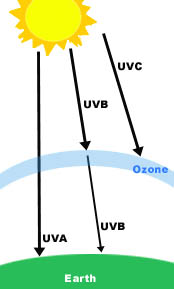
Why does ultraviolet light cause color to fade?Because of photodegradation.It is all about the chemical makeup of an object. The technical term for color fading is photodegradation. There are light absorbing color bodies called chromophores that are present in dyes. The color(s) we see are based upon these chemical bonds and the amount of light that is absorbed in a particular wavelength. Ultraviolet rays can break down the chemical bonds and thus fade the color(s) in an object - it is a bleaching effect. Some objects may be more prone to fading, such as dyed textiles and watercolors. Other objects may reflect the light more, which makes them less prone to fade.
|
|
||||
| The
Library of Congress >> Researchers >> Science Reference Services August 23, 2010 |
Legal | External Link Disclaimer |
Contact Us |