Vaccines
Although progress has been made in the last 10 years toward developing malaria vaccines, there is currently no effective malaria vaccine on the market.
Barriers to Developing a Malaria Vaccine
The development of a malaria vaccine has faced several obstacles: the lack of a traditional market, few developers, and the technical complexity of developing any vaccine against a parasite.
Malaria parasites have a complex life cycle, and there is poor understanding of the complex immune response to malaria infection. Malaria parasites are also genetically complex, producing thousands of potential antigens. Unlike the diseases for which we currently have effective vaccines, exposure to malaria parasites does not confer lifelong protection. Acquired immunity only partially protects against future disease, and malaria infection can persist for months without symptoms of disease.
RTS,S/AS01 Vaccine Candidate
More than a dozen vaccine candidates are now in clinical development, and one, GlaxoSmithKline Biologicals’ RTS,S, is in Phase III clinical testing—the first malaria vaccine candidate to advance this far.

KEMRI Clinical Officer Paul Ogai reviews a prospective participant's vaccination card during trial enrollment at the KEMRI/CDC site in Kenya. (Courtesy Alan Rubin, KEMRI)
Children ages 5-17 months were enrolled in the trial at 11 sites in seven African countries. CDC, in collaboration with the Kenya Medical Research Institute, led the trial at one site in western Kenya.
The trial’s first results, made available in October 2011, are a promising advance in development of a malaria vaccine for African children, which, if successful, could save hundreds of thousands of lives. The RTS,S vaccine reduced clinical and severe cases of malaria by half in children who received the vaccine.
Notably, the vaccine provided this protection in settings where there is ongoing use of other effective malaria prevention and treatment interventions: bed nets, antimalarial drugs, indoor residual insecticide spraying to prevent mosquito-borne transmission, and drugs to protect pregnant women and their newborns from malaria's adverse effects.
Still to come are analyses of how well the RTS,S/AS01 vaccine works in young infants (aged 6-12 weeks) when provided with their routine childhood immunizations, and how long the vaccine is protective. These results are expected in 2012 and 2014, respectively, and will be critical to understanding how the vaccine may be used to control malaria.
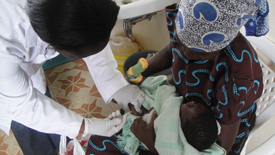
Nurse Dinah Mauti Maragwa gives malaria candidate vaccine to an infant at the Siaya KEMRI/CDC Malaria Vaccine Trial Site in Kenya. Courtesy: Alice Onsase and Kevin Shikanga, KEMRI/CDC
The world’s leading global health organizations have developed the Malaria Vaccine Technology Roadmap for accelerating development of a highly effective malaria vaccine.
The roadmap has two goals:
- Develop and license a first-generation vaccine by 2015 that reduces the risk of severe malaria disease and death by 50% and that protects longer than one year.
- Develop a malaria vaccine by 2025 that would have a protective efficacy of more than 80 percent against clinical disease and that would provide protection for longer than four years.
The recently released RTS,S results show we are on track to meeting that first goal. To meet the second will required a second-generation RTS,S vaccine or a different vaccine altogether.
Get email updates about Malaria
To receive email updates about this page, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
MS A-06
Atlanta, GA 30333 - Health care providers needing assistance with diagnosis or management of suspected cases of malaria should call the CDC Malaria Hotline:
770-488-7788 or 855-856-4713 toll-free
(M-F, 9am-5pm, eastern time). - Emergency consultation after hours, call:
770-488-7100
and request to speak with a CDC Malaria Branch clinician. - malaria@cdc.gov


