Carbon Sequestration
Why it Matters: Underground carbon sequestration is a technique in which one of the primary greenhouse gases, carbon dioxide (CO2), is removed from the atmosphere by injecting it into subsurface salt acquifers. This is a key potential global warming mitigation strategy.
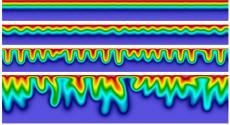
Key Challenges: A variety of geochemical processes can affect the mechanism of CO2 dissolution and the volume of CO2 that can be stored - the key result determining whether the strategy is effective or not. Simulation is the only way to study the detailed effects of geological flow, gravitational instability, rock heterogeneity, and brine salinity. These multicomponent, multiphase simulations must be carried out at high resolution and over enormous timeframes.
Accomplishments: A proven parallel, structured-grid, Adaptive Mesh Refinement (AMR) method has been applied to porous media flow to simulate with high accuracy the dissolution-diffusion-convection processes involved. The method was developed using NERSC's Cray XT4 ("Franklin") system and shows good scalability up to thousands of processors. Studies that routinely use up to 2,000 processors on Franklin have revealed how the onset time of convection and long-term stabilized mass flux vary with permeability, porosity, and effective diffusivity. Long-term behavior is convection-dominated and CO2 mass flux reaches a stabilized state that approaches a constant value at space and time scales of interest for geological storage.
Investigators: George Pau and John Bell, Lawrence Berkeley National Laboratory
More Information: See G. S. H. Pau, J. B. Bell, K. Pruess, A. S. Almgren, M. J. Lijewski, K. Zhang, "High resolution simulation and characterization of density-driven flow in CO2 storage in saline aquifers'', Advances in Water Resources, 33(4):443-455, 2010 and http://ccse.lbl.gov