About FDA
FY 2001 PDUFA Performance Report
Printable version of this report
Table of Contents
Original New Product Applications
Resubmitted New Product Applications
Procedural and Processing Goals
Appendices:
APPENDIX A:PDUFA II Performance Goals (FY 1998 - FY 2002)
APPENDIX B: List of Approved Applications
Executive Summary
In 1992, Congress enacted the Prescription Drug User Fee Act (PDUFA). PDUFA provided FDA with additional resources to hire more medical and scientific reviewers to conduct premarket re-views as well as support staff and field investigators to speed up the application review process for human drug and biological products. In 1997, after a successful first five years, Congress reauthorized the program for five additional years. With this reauthorization (PDUFA II) came higher expectations for reviews and additional goals intended to improve FDA's responsiveness to and communication with industry sponsors during the early years of drug development. FDA has been able to respond more rapidly to new drug and biologic applications without compro-mising review quality. For the consumer, this has meant more products available more quickly.
All of the original applications submitted during FY 2000 have been reviewed and acted upon, and final performance can now be reported. Only a preliminary performance assessment on ap-plications submitted during FY 2001 is possible at this time. FDA exceeded all the review per-formance goals for original and resubmitted new drug and biological applications and for efficacy and manufacturing supplements submitted in FY 2000. Although it is too early to report final results, the Agency appears to be meeting or exceeding all the review goals for FY 2001 submis-sions. The Agency also exceeded three of the six "procedural and processing" goals designed to improve its responsiveness to sponsors requests during the early phases of drug development (detailed results of performance on procedural and processing goals are on pages 16 and 17).
Notwithstanding these successes, the Agency has encountered challenges in trying to meet the PDUFA II goals. The fees the Agency has collected have been significantly less than expected due to the reduced number of new drug applications and the increased proportion of applications where fees are waived. Yet the number of goal-driven tasks for which the Agency collects no fees have increased substantially under PDUFA II.
The Agency will continue to work with the industry, the Congress, and all other stakeholders on a reauthorization of the PDUFA program that will continue to bring benefits to American consum-ers by bringing important new therapies to market quickly without compromising scientific re-view standards.
Introduction
In 1992, Congress passed the Prescription Drug User Fee Act (PDUFA). PDUFA authorized FDA to collect fees from companies that produce human drug and biological products. The original PDUFA had a five-year life; it ended in 1997, the same year Congress passed the FDA Modernization Act (FDAMA). FDAMA contained a five-year reauthorization of PDUFA (PDUFA II).
PDUFA requires FDA to submit two annual reports to Congress for each fiscal year during which fees are collected: 1) a performance report due within 60 days of the end of the fiscal year, and 2) a financial report due within 120 days of the end of the fiscal year. This document fulfills the first of these requirements for Fiscal Year 2001.
PDUFA provides FDA with additional revenue to hire more reviewers and support staff and upgrade its information technology to speed up the application review process for pharmaceutical and biological products without compromising review quality.
In consultation with industry and the Congress, FDA agreed to meet a set of review performance goals that become more stringent each year. These goals applied to the review of original new product applications, resubmissions of original applications, and supplements to approved applications. FDA met or exceeded every PDUFA I performance goal and has met or exceeded nearly every PDUFA II performance goal.
Under PDUFA II, the review goals continue to shorten. By 2002, the PDUFA II goals call for FDA to review and act on 90 percent of:
- Priority new drug and biological product applications and efficacy supplements (i.e., for products providing significant therapeutic gains) within 6 months;
- Standard new drug and biological product applications and efficacy supplements within 10 months;
- Manufacturing supplements within 6 months, and those requiring prior approval within 4 months;
- Class 1 resubmissions of original applications within 2 months, and Class 2 resubmissions of original applications within 6 months.
In addition, PDUFA II added a new set of procedural goals intended to improve FDA's responsiveness to and communication with industry sponsors during the early years of drug development. These goals specify timeframes for activities such as scheduling meetings and responding to various sponsor requests. Whereas PDUFA's original intent was to speed up the review process, PDUFA II's intent is to speed up the entire drug development process.
Outcomes
In FY 2001 the Agency exceeded all its review performance goals of PDUFA II1 despite the goals becoming still more challenging. However, some strains in the program became evident. While some aspects of workload decreased, others, notably those involving pre-submission meetings, increased, and the Agency was unable to achieve all of its procedural and processing performance goals (e.g., goals associated with formal meetings with sponsors).
Fewer Fee-Paying Applications: Original applications and efficacy supplements are the most important component of user-fee revenues. Not only do they generate one-third of the total amount of fees collected, but they also determine the amount to be generated by product and establishment fees, which are set to generate the other two-thirds of the fee revenue. The number of original new product applications submitted and filed each year under PDUFA increased steadily in the early years from 88 in FY 1993 to 133 in FY 1997.
From FY 1997 to FY 2000, the growth leveled off, and in FY 2001, application submissions and filings dropped substantially. When applications are submitted, they are reviewed for completeness before they are filed. Even though only three applications were refused for filing, FDA filed 25 percent fewer applications last year than the previous year. The decrease was especially significant for priority applications -- those that represent significant therapeutic gains. The number of priority applications filed last year was down more than 60% from the previous year and was less than half the level of any year since FY 1997.

The number of efficacy supplements also decreased last year, from a level of 187 in FY 2000 to 168, a decrease of 10%. Priority efficacy supplements dropped from 20 in FY 2000 to 9 in FY 2001.
Other PDUFA Activities: Although the number of applications the Agency filed dropped last year, pre-submission activity increased. The number of pre-submission meetings requested, scheduled, and transcribed increased from the previous year. Pre-submission meetings play an important role in improving communications between the industry and the Agency and speeding the drug development process, but they consume a significant proportion of the Agency's resources that would otherwise be allocated to the review of applications. The Agency received 1,471 requests for formal meetings from sponsors and scheduled 1,361 meetings. These numbers are increases of 24% and 21% respectively over the previous year.

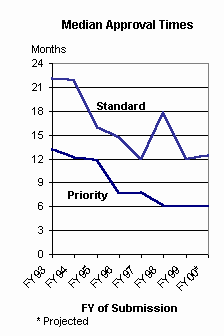
Approval Times Remain Short: Total approval time is the time from the initial submission of an original application to the issuance of an approval letter for that application. It includes both FDA's review time and the time the sponsor spends answering deficiencies noted by FDA and can encompass several review 'cycles.' Not all applications receive approval letters. While PDUFA specifies review-time goals and quicker reviews tend to produce quicker approvals, the quality and completeness of the new drug application and the public health priority of the product also have a significant impact on time to approval.
Median total approval time for priority applications submitted in FY 2000 remained at 6 months, less than half what it was in the early PDUFA years. Median approval time for standard applications increased slightly from an estimated 12.0 months for FY 1999 submissions to 12.5 months for FY 20002.
Report on PDUFA Goals
This report updates the Agency's review performance on the FY 2000 application submissions and evaluates its performance in reviewing FY 2001 application submissions and meeting other PDUFA II goals. All of the original applications submitted during FY 2000 have been reviewed and acted upon, and final performance can now be reported (One standard submission was acted on after September 30, 2001, but it is included in the performance statistics). Only a preliminary performance assessment on applications submitted during FY 2001 is possible at this time. For submission categories with a 10- or 12-month review goal, it is too early to measure review per-formance. For those submission categories with a review goal that is shorter than 10 months, per-formance on submissions received early in the fiscal year provides an early-indicator of final re-view performance. Unless otherwise noted, all performance data in this section are as of September 30, 2001.
FDA's Center for Biologics Evaluation and Research (CBER) has changed from counting Product License Applications (PLAs) and Establishment License Applications (ELAs) separately to com-bining them as Biologic License Applications (BLAs). This report shows CBER's workload and performance on PLAs and BLAs only (i.e., Product Applications). To simplify notation, it uses BLA as a generic term for both BLAs and PLAs. Original and resubmitted ELAs have been dropped, both from workload counts and performance measurements. These new counts are re-flected in the workload and performance data for the PDUFA I years, so trends into PDUFA II are consistent.
Report on PDUFA Goals: Original New Product Applications
Goal --Review and act upon complete original NDAs and BLAs
The table below summarizes the annually decreasing review-time goals for original New Drug Applications (NDAs) and BLAs under PDUFA II. Over the five-year period, the goal of reviewing 90 percent of priority applications in six months remains constant. For standard applications, the review-time goals drop over the five-year period. For applications filed in FY 98, the goal was to review 90 percent in 12 months; for FY 02 applications, the goal is to review 90 percent in 10 months. For standard applications filed in FY 2001, the goal was to review 90 percent in 12 months and 70 percent in 10 months. The statute allows three additional months for review of original NDA and BLA submissions that involve major amendments received within the last three months of their usual review intervals.
| Goals | On-Time Performance by Submission Year | |||||
|---|---|---|---|---|---|---|
| FY 98 | FY 99 | FY 00 | FY 01 | FY 02 | ||
| Priority | 6 months | 90% on time | 90% | 90% | 90% | 90% |
| Standard | 12 months 10 months | 90% | 90% 30% | 90% 50% | 90% 70% | 90% |
Workload
The following table shows the number of original NDAs and BLAs filed in each of the last five years. The count of FY 01 submissions assumes that all submissions received in the last two months of FY 01 are filed. When FDA files a submission, it is deemed "complete" by PDUFA definition. FDA makes a filing decision within 60 days of an original application's receipt. All calculations of PDUFA review times are made, however, from the original receipt date of the filed application.
| Original submissions filed (Priority/Standard): | |||||
|---|---|---|---|---|---|
| FY 97 | FY 98 | FY 99 | FY 00 | FY 013 | |
NDAs | 117 (25/92) | 109 (30/79) | 121 (30/91) | 121 (29/92) | 92 (10/82) |
BLAs | 16 (3/13) | 12 (8/4) | 6 (1/5) | 13 (4/9) | 9 (3/6) |
PDUFA Total | 133 28/105 | 121 (38/83) | 127 (31/96) | 134 (33/101) | 101 (13/88) |
NMEs4 | 42 (19/23) | 41 (16/25) | 32 (17/15) | 34 (8/26) | |
Performance
FY 2000 Submissions
For FY 2000 applications, FDA met the 6-month review goal for 32 of 33 priority submissions. One priority submission was late. FDA met the 12-month goal for standard submissions for 98 of the 101 standard submissions reviewed. Eighty-one percent of all standard applications and 65 percent of the NMEs and BLAs were reviewed and acted upon within 10 months, exceeding the 50 percent review goal in both cases.
| FY 00 Submissions | Reviewed and acted upon | Number on time | Percent on time | ||
|---|---|---|---|---|---|
| Priority | 6 month goal | All Applications | 33 | 32 | 97 |
| NMEs & BLAs | 21 | 20 | 95 | ||
| Standard | 12 month goal | All Applications | 101 | 98 | 97 |
| NMEs & BLAs | 24 | 23 | 96 | ||
| 10 month goal | All Applications | 101 | 82 | 81 | |
| NMEs & BLAs | 24 | 15 | 62 | ||
FY 2001 Submissions
While it is too early to report meaningful review performance statistics for applications submitted in FY 2001, all priority applications that have been reviewed have met the 6 month review goal, and all standard applications that have been reviewed have met the 10 month review goal.
| FY 01 Submissions | Reviewed and acted upon | Number on time | Percent on time | ||
|---|---|---|---|---|---|
| Priority | 6 month goal | All Applications | 8 | 8 | 100 |
| NMEs & BLAs | 7 | 7 | 100 | ||
| Standard | 12 month goal | All Applications | Too early to report meaningful review performance statistics. Ten standard applications have been reviewed and acted upon, all within 10 months. | ||
| NMEs & BLAs | |||||
| 10 month goal | All Applications | ||||
| NMEs & BLAs | |||||
Report on PDUFA Goals: Resubmitted New Product Applications
Goal--Review and act upon resubmitted NDAs and BLAs.
A resubmission is a firm's response after an FDA action of "approvable," "not approvable," or "complete response" on an application. The applicable performance goal for a resubmission is determined by the year in which the resubmission itself is received, rather than its original application's year of submission. The definitions of Class 1 and Class 2 resubmissions can be found at the end of Appendix A.
| Goals | On-Time Performance by Resubmission Year | |||||
|---|---|---|---|---|---|---|
| FY 98 | FY 99 | FY 00 | FY 01 | FY 02 | ||
| Class 1 | 6 months 4 months 2 months | 90% on time
30% |
90% 50% |
90% 70% |
90% |
90% |
| Class 2 | 6 months | 90% | 90% | 90% | 90% | 90% |
Workload
| Workload -- Resubmissions received [Total (Class 1/Class 2)] | |||||
|---|---|---|---|---|---|
| FY 97 | FY 98 | FY 99 | FY 00 | FY 01 | |
| of Original NDAs | 82 | 50 (19/31) | 63 (17/46) | 80 (25/55) | 61 (27/34) |
| of Original BLAs | 8 | 21 (5/16) | 14 (2/12) | 9 (1/8) | 15 (6/9) |
| PDUFA Total | 90 | 71 (24/47) | 77 (19/58) | 89 (26/63) | 76 (33/43) |
Performance
FY 2000 Submissions:
All 26 Class 1 resubmissions received in FY 2000 were reviewed and acted upon within 4 months, 25 of them within 2 months. All but one of the 63 Class 2 resubmissions were reviewed and acted upon within 6 months. Review performance on both classes of FY 00 resubmissions exceeded the PDUFA review goals.
| FY 00 Resubmissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Class 1 | 4 months 2 months | 26 | 26 25 | 100 96 |
| Class 2 | 6 months | 63 | 62 | 98 |
FY 2001 Submissions:
As of September 30, 2001, 24 FY 2001 Class 1 resubmissions had been reviewed and acted upon. Twenty-two of these met the 2-month goal. All of the Class 2 resubmissions that have been reviewed and acted upon have met the 6-month goal. With 9 Class 1 and 15 Class 2 resubmissions still pending and not overdue, it is too early to make a final performance determination, but current on-time performance for both classes of resubmissions exceeds the goals.
| FY 01 Resubmissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Class 1 | 2 months | 24 | 22 | 92 |
| Class 2 | 6 months | 28 | 28 | 100 |
Report on PDUFA Goals: Efficacy Supplements
Goal -- Review and act upon complete efficacy supplements to NDAs and BLAs
The table below summarizes the annually decreasing review-time goals for efficacy supplements to NDAs and BLAs under PDUFA II. Review goals for efficacy supplements follow the same progression as the review goals for original NDAs and BLAs. Over the five-year period, the goal of reviewing 90 percent of priority efficacy supplements in six months remains constant. For standard efficacy supplements, the review-time goals drop over the five-year period. For FY 98 submissions, the goal was to review 90 percent in 12 months; for FY 02 submissions, the goal is to review 90 percent in 10 months. For standard efficacy supplements received in FY 2001, the goal was to review 90 percent in 12 months and 70 percent in 10 months.
| Goals | On-Time Performance by Submission Year | |||||
|---|---|---|---|---|---|---|
| FY 98 | FY 99 | FY 00 | FY 01 | FY 02 | ||
| Priority | 6 months | 90% on time | 90% | 90% | 90% | 90% |
| Standard | 12 months 10 months | 90% | 90% 30% | 90% 50% | 90% 70% | 90% |
Workload
| Workload -- Efficacy supplements filed (Priority / Standard): | |||||
|---|---|---|---|---|---|
| FY 97 | FY 98 | FY 99 | FY 00 | FY 014 | |
| to NDAs | 146 (10/136) | 126 (10/116) | 135 (15/120) | 175 (18/157) | 152 (7/145) |
| to BLAs | 15 (3/12) | 10 (1/9) | 10 (2/8) | 12 (2/10) | 16 (2/14) |
| PDUFA total | 161 (13/148) | 136 (11/125) | 145 (17/128) | 187 (20/167) | 168 (9/159) |
Performance
FY 2000 Submissions
All 20 of the priority efficacy supplements submitted in FY 2000 were reviewed and acted upon within the 6 month review goal. On-time performance was 100 percent which exceeded the 90 percent goal.
All but one of the standard efficacy supplements were reviewed and acted upon within 12 months and 91 percent were reviewed within 10 months. This performance exceeds the FY 2000 goals of 90 percent and 50 percent respectively.
| FY 00 Submissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Priority | 6 months | 20 | 20 | 100 |
| Standard | 12 months 10 months | 167 | 166 152 | 99 91 |
FY 2001 Submissions
Four of the nine priority efficacy supplements submitted in FY 2001 have been reviewed and acted upon. All have met the 6-month review goal. Only 19 of the 159 standard efficacy supplements have been reviewed. All of these have met the 10-month review goal.
| FY 01 Submissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Priority | 6 months | 4 | 4 | 100 |
| Standard | 12 months 10 months | Too early to report meaningful review performance statistics. Nineteen standard efficacy supplements have been reviewed and acted upon, all within 10 months | ||
Report on PDUFA Goals: Manufacturing Supplements
Goal -- Review and act upon complete manufacturing supplements to NDAs and BLAs
The review performance goals for manufacturing supplements that do not require FDA approval before the changes they specify can be enacted do not change over the five years of PDUFA II. For manufacturing supplements that do require FDA's approval, the goal times decrease from 6 months for FY 1998 submissions to 4 months for FY 2002 submissions.
| Goals | On-Time Performance by Submission Year | |||||
|---|---|---|---|---|---|---|
| FY 98 | FY 99 | FY 00 | FY 01 | FY 02 | ||
| Prior approval not required | 6 months | 90% on time | 90% | 90% | 90% | 90% |
| Prior approval required | 6 months 4 months | 90% | 90% 30% | 90% 50% | 90% 70% | 90% |
Workload
| Workload -- Manufacturing supplements filed (Prior Approval / No Prior Approval): | |||||
|---|---|---|---|---|---|
| FY 97 | FY 98 | FY 99 | FY 00 | FY 01 4 | |
| to NDAs | 1,262 | 1,463 | 1,459 (900/559) | 1,438 (684/754) | 1,474 (585/889) |
| to BLAs | 338 | 371 | 477 (259/218) | 587 (239/348) | 595 (181/414) |
| PDUFA total | 1,600 | 1,834 | 1,936 (1,159/777) | 2,025 (923/1,102) | 2,069 (766/1,303) |
Performance
FY 2000 Submissions
Ninety-eight percent of the manufacturing supplements that did not require prior FDA approval submitted in FY 2000 were reviewed within 6 months. That level of performance exceeded the 90 percent on-time review goal.
Ninety-seven percent of the manufacturing supplements that required prior FDA approval submitted in FY 2000 also were reviewed within 6 months. Seventy-nine percent of these were reviewed within 4 months. That level of performance exceeded FY 2000's goals of 90 percent and 50 percent respectively.
| FY 00 Submissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Prior approval not required | 6 months | 1102 | 1077 | 98 |
| Prior approval required | 6 months 4 months | 923 | 897 725 | 97 79 |
FY 2001 Submissions
As of September 30, 2001, almost 64 percent of the manufacturing supplements that do not require prior approval, and 72 percent of those that do require prior approval had been reviewed. Ninety-nine percent of both categories of manufacturing supplements had been reviewed within 6 months, and 89 percent of those requiring prior approval had been reviewed within 4 months. Although it is too early to make a final determination with only 67 percent of the submissions reviewed, performance in all categories is well above the FY 2001 review goals.
| FY 01 Submissions | Reviewed and acted upon | Number on time | Percent on time | |
|---|---|---|---|---|
| Prior approval not required | 6 months | 831 | 824 | 99 |
| Prior approval required | 6 months 4 months | 551 | 547 490 | 99 89 |
Report on PDUFA Goals: Procedural and Processing Goals
This section reports on a number of PDUFA II goals that had no precedent under PDUFA I. These goals relate to the IND phase of drug development and some aspects of the infrastructure of drug review. A detailed description of the goals, the annual performance targets, and definitions of terms can be found in Appendix A. This section reports on actions on items that occurred in FY 2001.
Meeting Management:
- Meeting Requests: Notify requestor of formal meeting in writing within 14 days of request.
- Scheduling Meetings: Schedule meetings within goal date (within 30 days of receipt of request for Type A meetings, 60 days for Type B meetings, and 75 days for Type C meetings). If the requested date for any of these types of meetings is greater than 30, 60, or 75 days, as appropriate, from the date the request is received by the Agency, the meeting date should be within 14 days of the requested date.
- Meeting Minutes: Agency prepared minutes, clearly outlining agreements, disagreements, issues for further discussion and action times will be available to sponsor within 30 calendar days of meeting.
| Total | Met Goal | Missed Goal5 | Pending Within Goal6 | % On Time7 | |||
|---|---|---|---|---|---|---|---|
| On-time Goal | 90% | ||||||
| Meeting Requests | CBER | 387 | 376 | 9 | 2 | ||
| CDER | 1084 | 942 | 123 | 19 | |||
| Combined | 1471 | 1318 | 132 | 21 | 91% | ||
| Scheduling Meetings | Type A | CBER | 22 | 18 | 2 | 2 | |
| CDER | 36 | 25 | 11 | 0 | |||
| Type B | CBER | 229 | 191 | 11 | 27 | ||
| CDER | 386 | 258 | 116 | 12 | |||
| Type C | CBER | 89 | 75 | 2 | 12 | ||
| CDER | 599 | 564 | 28 | 7 | |||
| All | CBER | 340 | 284 | 15 | 41 | ||
| CDER | 1021 | 847 | 155 | 19 | |||
| Combined | 1361 | 1131 | 170 | 60 | 87% | ||
| Meeting Minutes | CBER | 243 | 216 | 6 | 21 | ||
| CDER | 979 | 440 | 229 | 310 | |||
| Combined | 1222 | 656 | 235 | 331 | 74% | ||
Clinical Holds: Respond to sponsor's complete response to a clinical hold within 30 days of receipt
| Total | Met Goal | Missed Goal5 | Pending Within Goal6 | % On Time7 | ||
|---|---|---|---|---|---|---|
| On-time Goal | 90% | |||||
| CBER | 125 | 115 | 10 | 0 | ||
| CDER | 34 | 25 | 8 | 1 | ||
| Combined | 159 | 140 | 18 | 1 | 89% | |
Major Dispute Resolution: Respond to sponsor's appeal of decision within 30 days of receipt
| Total | Met Goal | Missed Goal5 | Pending Within Goal6 | % On Tim7 | ||
|---|---|---|---|---|---|---|
| On-time Goal | 90% | |||||
| CBER | 2* | 2* | 0 | 0 | ||
| CDER | 9 | 9 | 0 | 0 | ||
| Combined | 11 | 11 | 0 | 0 | 100% | |
Special Protocol Question Assessment and Agreement: Respond to sponsor's request for evaluation of protocol design within 45 days of receipt
| Total | Met Goal | Missed Goal5 | Pending Within Goal6 | % On Time7 | ||
|---|---|---|---|---|---|---|
| On-time Goal | 80% | |||||
| CBER | 1 | 1 | 0 | 0 | ||
| CDER | 120 | 88 | 18 | 14 | ||
| Combined | 121 | 89 | 18 | 14 | 83% | |
Notes on the FY 2000 PDUFA Performance Report to Congress
- This report uses the terms PDUFA I and PDUFA II to distinguish between the original Prescription Drug User Fee Act of 1992 and the Act as reauthorized and amended by the Food and Drug Administration Modernization Act of 1997 (FDAMA) respectively. Where no distinction is needed or where the reference is obvious, the term PDUFA is used.
- Although the last approvals for FY 2000 submissions (as well as for earlier years) have not yet occurred, the median statistic can be estimated from approvals to date and estimates of the percent of submissions that will ultimately be approved.
- The count of FY 2001 submissions assumes that all submissions received in the last two months of FY 2001 are filed. When FDA files a submission, it is deemed "complete" by PDUFA definition. FDA makes a filing decision within 60 days of an original application's receipt. All calculations of PDUFA review times are made, however, from the original receipt date of the filed application.
- The term NME in this report refers exclusively to NMEs that are NDAs. For FDAMA purposes, BLAs are considered to be equivalent to NMEs; however, workload and performance statistics for BLAs are reported separately. The counts of NMEs in the workload table are of 'discrete,' filed NMEs. CDER often receives multiple submissions for the same new molecular entity, for different dosage forms for example. All are initially designated as NMEs, but, when the first of the multiples is approved, the others are re-designated as non-NMEs. In FY 2001, CDER designated 38 filings as NMEs initially (8 priority, 30 standard). Only 34 of these are 'discrete' (8 priority, 26 standard).
- Includes those with late actions and those still pending whose goal date has passed and which have not had actions.
- Includes actions that are pending within goal, as well as those whose goal date has passed, but whose action status is deemed incomplete because the database had not been updated to reflect the action in time for this report.
- Actions pending were excluded from the calculation.
APPENDIX A: PDUFA Performance Goals, FY 1998 - FY 2002
The following list presents by fiscal year the performance measures set forth in the letters referenced in the Food and Drug Administration Modernization Act of 1997. The following chart lists the goals by fiscal year with appropriate goal measurement dates:
FIVE-YEAR REVIEW PERFORMANCE GOALS
| Fiscal Year 1998 | MEASUREMENT DATE |
|---|---|
| Review and act on 90 percent of standard original NDAs and PLA/BLAs filed during FY 98 within 12 months of receipt1. | 12 months after end of FY 1998 |
| Review and act on 90 percent of priority original NDAs and PLA/BLAs filed during FY 98 within 6 months of receipt.1 | 6 months after end of FY 1998 |
| Review and act on 90 percent of standard efficacy supplements filed during FY 98 within 12 months of receipt. | 12 months after end of FY 1998 |
| Review and act on 90 percent of priority efficacy supplements filed during FY 98 within 6 months of receipt. | 6 months after end of FY 1998 |
| Review and act on 90 percent of manufacturing supplements filed during FY 98 within 6 months of receipt. | 6 months after end of FY 1998 |
| Review and act on 90 percent of resubmitted original applications received during FY 98 within 6 months of receipt, and review and act on 30 percent of Class 1 resubmitted original applications within 2 months of receipt. | 6 months after end of FY 1998 |
1The goal letter allows three additional months for review of original NDA, PLA, or BLA submissions that involve major amendments within the last three months of their usual review interval. In these cases, the measurement dates shown in this Appendix move forward by 3 months.
| Fiscal Year 1999 | MEASUREMENT DATE |
|---|---|
| 1. Review and act on 90 percent of standard original NDAs and PLA/BLAs filed during FY 99 within 12 months of receipt and review and act on 30 percent within 10 months of receipt.1 | 12 months after end of FY 99 |
| 2. Review and act on 90 percent of priority original NDAs and PLA/BLAs filed during FY 99 within 6 months of receipt.1 | 6 months after end of FY 99 |
| 3. Review and act on 90 percent of standard efficacy supplements filed during FY 99 within 12 months of receipt and review and act on 30 percent within 10 months of receipt. | 12 months after end of FY 99 |
| 4. Review and act on 90 percent of priority efficacy supplements filed during FY 99 within 6 months of receipt. | 6 months after end of FY 99 |
| 5. Review and act on 90 percent of manufacturing supplements filed during FY 99 within 6 months of receipt and review and act on 30 percent of manufacturing supplements requiring prior approval within 4 months of receipt. | 6 months after end of FY 99 |
| 6. Review and act on 90 percent of Class 1 resubmitted original applications received during FY 99 within 4 months of receipt, and review and act on 50 percent within 2 months of receipt. | 4 months after end of FY 99 |
| 7. Review and act on 90 percent of Class 2 resubmitted original applications received during FY 99 within 6 months of receipt. | 6 months after end of FY 99 |
1The goal letter allows three additional months for review of original NDA, PLA, or BLA submissions that involve major amendments within the last three months of their usual review interval. In these cases, the measurement dates shown in this Appendix move forward by 3 months.
| Fiscal Year 2000 | MEASUREMENT DATE |
|---|---|
| 1. Review and act on 90 percent of standard original NDAs and PLA/BLAs filed during FY 2000 within 12 months of receipt and review and act on 50 percent within 10 months of receipt.1 | 12 months after end of FY 2000 |
| 2. Review and act on 90 percent of priority original NDAs and PLA/BLAs filed during FY 2000 within 6 months of receipt.1 | 6 months after end of FY 2000 |
| 3. Review and act on 90 percent of standard efficacy supplements filed during FY 2000 within 12 months of receipt and review and act on 50 percent within 10 months of receipt. | 12 months after end of FY 2000 |
| 4. Review and act on 90 percent of priority efficacy supplements filed during FY 2000 within 6 months of receipt. | 6 months after end of FY 2000 |
| 5. Review and act on 90 percent of manufacturing supplements filed during FY 2000 within 6 months of receipt and review and act on 50 percent of manufacturing supplements requiring prior approval within 4 months of receipt. | 6 months after end of FY 2000 |
| 6. Review and act on 90 percent of Class 1 resubmitted original applications received during FY 2000 within 4 months of receipt, and review and act on 70 percent within 2 months of receipt. | 4 months after end of FY 2000 |
| 7. Review and act on 90 percent of Class 2 resubmitted original applications received during FY 2000 within 6 months of receipt. | 6 months after end of FY 2000 |
1The goal letter allows three additional months for review of original NDA, PLA, or BLA submissions that involve major amendments within the last three months of their usual review interval. In these cases, the measurement dates shown in this Appendix move forward by 3 months.
| Fiscal Year 2001 | MEASUREMENT DATE |
|---|---|
| 1. Review and act on 90 percent of standard original NDAs and PLA/BLAs filed during FY 2001 within 12 months of receipt and review and act on 70 percent within 10 months of receipt.1 | 12 months after end of FY 2001 |
| 2. Review and act on 90 percent of priority original NDAs and PLA/BLAs filed during FY 2001 within 6 months of receipt.1 | 6 months after end of FY 2001 |
| 3. Review and act on 90 percent of standard efficacy supplements filed during FY 2001 within 12 months of receipt and review and act on 70 percent within 10 months of receipt. | 12 months after end of FY 2001 |
| 4. Review and act on 90 percent of priority efficacy supplements filed during FY 2001 within 6 months of receipt. | 6 months after end of FY 2001 |
| 5. Review and act on 90 percent of manufacturing supplements filed during FY 2001 within 6 months of receipt and review and act on 70 percent of manufacturing supplements requiring prior approval within 4 months of receipt. | 6 months after end of FY 2001 |
| 6. Review and act on 90 percent of Class 1 resubmitted original applications received during FY 2001 within 2 months of receipt. | 2 months after end of FY 2001 |
| 7. Review and act on 90 percent of Class 2 resubmitted original applications received during FY 2001 within 6 months of receipt. | 6 months after end of FY 2001 |
1The goal letter allows three additional months for review of original NDA, PLA, or BLA submissions that involve major amendments within the last three months of their usual review interval. In these cases, the measurement dates shown in this Appendix move forward by 3 months.
| Fiscal Year 2002 | MEASUREMENT DATE |
|---|---|
| 1. Review and act on 90 percent of standard original NDAs and PLA/BLAs filed during FY 2002 within 10 months of receipt.1 | 10 months after end of FY 2002 |
| 2. Review and act on 90 percent of priority original NDAs and PLA/BLAs filed during FY 2002 within 6 months of receipt.1 | 6 months after end of FY 2002 |
| 3. Review and act on 90 percent of standard efficacy supplements filed during FY 2002 within 10 months of receipt. | 10 months after end of FY 2002 |
| 4. Review and act on 90 percent of priority efficacy supplements filed during FY 2002 within 6 months of receipt. | 6 months after end of FY 2002 |
| 5. Review and act on 90 percent of manufacturing supplements filed during FY 2002 within 6 months of receipt and review and act on 90 percent of manufacturing supplements requiring prior approval within 4 months of receipt. | 6 months after end of FY 2002 |
| 6. Review and act on 90 percent of Class 1 resubmitted original applications received during FY 2002 within 2 months of receipt. | 2 months after end of FY 2002 |
| 7. Review and act on 90 percent of Class 2 resubmitted original applications received during FY 2002 within 6 months of receipt. | 6 months after end of FY 2002 |
1The goal letter allows three additional months for review of original NDA, PLA, or BLA submissions that involve major amendments within the last three months of their usual review interval. In these cases, the measurement dates shown in this Appendix move forward by 3 months.
NEW MOLECULAR ENTITY (NME) PERFORMANCE GOALS
The performance goals for standard and priority original NMEs will be the same as for all of the original NDAs but will be reported separately.
For biological products, for purposes of this performance goal, all original PLA/BLAs will be considered to be NMEs.
PROCEDURAL AND PROCESSING GOALS
| Performance Area | Agency Activity | Performance Goal | Performance Level |
|---|---|---|---|
| Meeting Management | Meeting Requests -- Notify requester of formal meeting in writing (date, time, place, and participants) | within 14 days of receipt of request | FY 1999 requests -- 70% on time FY 2000 -- 80% on time FY2001 and on -- 90% on time |
| Scheduling Meetings -- Schedule meetings within goal date or within 14 days of requested date if longer than goal date. | Type A Meetings within 30 days of receipt of request | FY 1999 requests -- 70% on time FY 2000 -- 80% on time FY2001 and on -- 90% on time | |
| Type B Meetings within 60 days of receipt of request | |||
| Type C Meetings within 75 days of receipt of request | |||
| Meeting Minutes -- Agency prepared minutes, clearly outlining agreements, disagreements, issues for further discussion and action times will be available to sponsor | within 30 calendar days of meeting | FY 1999 meetings -- 70% on time FY 2000 -- 80% on time FY2001 and on -- 90% on time | |
| Clinical Holds | Response to sponsor's complete response to a clinical hold | within 30 days of receipt of sponsor's response | FY 1998 -- 75% on time FY 1999 and on -- 90% on time |
| Major Dispute Resolution | Response to sponsor's appeal of decision | within 30 days of receipt of sponsor's appeal | FY 1999 -- 70% on time FY 2000 -- 80 % on time FY 2001 and on -- 90% on time |
| Special Protocol Question Assessment and Agreement | Response to sponsor's request for evaluation of protocol design | within 45 days of receipt of protocol and questions | FY 1999 -- 60% on time FY 2000 -- 70% on time FY 2001 -- 80% on time FY 2002 -- 90% on time |
| Electronic Applications and Submissions | Paperless Application Processing | Agency to develop and update information systems to allow paperless receipt and processing of INDs, human drug applications, and related submissions by end of FY 2002. | |
| Additional Procedures | Simplification of Action Letters | Centers to amend regulations and processes to provide for issuance of 'Approval' (AP) or 'Complete Response' (CR) action letters. | |
| Sponsor Notification of Deficiencies in Applications | Centers to notify sponsors of deficiencies via 'information request' (IR) when each discipline has finished its initial review. | ||
Definition of Terms:
- The term "review and act on" is understood to mean the issuance of a complete action letter after the complete review of a filed complete application. The action letter, if it is not an approval, will set forth in detail the specific deficiencies and, where appropriate, the actions necessary to place the application in condition for approval.
- A major amendment to an original application submitted within three months of the goal date extends the goal date by three months. Only one extension is allowed for an application.
- A resubmitted original application is a complete response to an action letter addressing all identified deficiencies.
- Class 1 resubmitted applications are applications resubmitted after a complete response letter (or a not approvable or approvable letter) that include the following items only (or combinations of these items):
- Final printed labeling
- Draft labeling
- Safety updates submitted in the same format, including tabulations, as the original safety submission with new data and changes highlighted (except when large amounts of new information including important new adverse experiences not previously reported with the product are presented in the resubmission)
- Stability updates to support provisional or final dating periods
- Commitments to perform Phase 4 studies, including proposals for such studies
- Assay validation data
- Final release testing on the last 1-2 lots used to support approval
- A minor reanalysis of data previously submitted to the application (determined by the agency as fitting the Class 1 category)
- Other minor clarifying information (determined by the Agency as fitting the Class 1 category)
- Other specific items may be added later as the Agency gains experience with the scheme and will be communicated via guidance documents to industry.
- Class 2 resubmissions are resubmissions that include any other items, including any item that would require presentation to an advisory committee.
- A Type A Meeting is a meeting that is necessary for an otherwise stalled drug development program to proceed (a "critical path" meeting).
- A Type B Meeting is a 1) pre-IND, 2) end of Phase 1 (for Subpart E or Subpart H or similar products) or end of Phase 2/pre-Phase 3, or 3) a pre- NDA/PLA/BLA meeting. Each requester should usually only request 1 each of these Type B meetings for each potential application (NDA/PLA/BLA) (or combination of closely related products, i.e., same active ingredient but different dosage forms being developed concurrently).
- A Type C Meeting is any other type of meeting.
APPENDIX B: List of Approved Applications
Tables(Priority and Standard NDA/BLA Approvals by Fiscal Year):
FY 2000 Priority | FY 2000 Standard | FY 1999 Standard | FY 1998 Standard | FY 1997 Standard
This appendix updates the detailed review histories of the NDAs and PLA/BLAs submitted and approved under PDUFA. It shows approvals of all PDUFA-related submissions that took place in FY 01 as well as FY 00 approvals of FY 00 submissions. Earlier PDUFA approvals were listed in previous performance reports.
The following two tables summarize the review histories for all approved applications submitted from FY 96 through FY 00. The tables show the average first review, second review, and approval times. Note that times are in months, not all applications required a second review, and some required more than two reviews. The mean total approval times shown in the tables will increase in the future as additional applications are approved.
Approved Priority NDAs/BLAs
| 1st Review | 2nd Review | Total Approval Time | ||||
|---|---|---|---|---|---|---|
| Receipt Cohort | N | FDA Review | n | Sponsor Response | FDA Review | |
| FY96 | 32 | 7.4 | 14 | 5.4 | 3.8 | 13.4 |
| FY97 | 23 | 6.3 | 10 | 4.4 | 3.6 | 9.5 |
| FY98 | 30 | 6.1 | 12 | 1.5 | 2.7 | 8.4 |
| FY99 | 25 | 6.3 | 7 | 1.6 | 2.1 | 7.3 |
| FY00 | 20 | 5.9 | 5 | 2.1 | 3.4 | 7.4 |
Approved Standard NDAs/BLAs
| 1st Review | 2nd Review | Total Approval Time | ||||
|---|---|---|---|---|---|---|
| Receipt Cohort | n | FDA Review | n | Sponsor Response | FDA Review | |
| FY96 | 73 | 11.9 | 40 | 4.1 | 4.1 | 17.1 |
| FY97 | 83 | 11.6 | 36 | 5.3 | 3.8 | 15.9 |
| FY98 | 63 | 11.4 | 39 | 5.0 | 4.8 | 18.3 |
| FY99 | 62 | 10.6 | 26 | 2.8 | 3.5 | 14.0 |
| FY00 | 46 | 10.5 | 15 | 1.9 | 3.0 | 12.1 |
The remainder of this appendix shows the individual review histories. Approvals are grouped by submission year and priority designation and listed in order of total approval time. Review histories of all other PDUFA submissions approved prior to FY 99 can be found in the appendices of the earlier PDUFA Performance Reports which are available on FDA's web site (FDA Home)
Terms and Coding Used In Tables
| (+) FY 01 approval of a FY 01 submission. These were not included in earlier PDUFA performance reports and are included here for completeness. | |
| (**) Major amendment was received within 3 months of the action due date, which extended the review timeframes by 3 months. | |
| Action Codes: | AE = Approvable AP = Approved NA = Not Approvable RL = Complete Response WD = Withdrawn |
Table 1
FY 2000 Priority NDA and BLA Submissions Approved in FY 00 (+) and FY 01
| Generic Name | Sponsor | Approval Time (Months) | Review Goal Met | ||
|---|---|---|---|---|---|
| Total Time | Resubmissions (if necessary) | ||||
+ | LOPINAVIR; RITONAVIR (ORAL SOLUTION) | Abbott Labs | 3.5 | Y | |
+ | LOPINAVIR; RITONAVIR (CAPSULE) | Abbott Labs | 3.5 | Y | |
| MESALAMINE | Axcan Scandipharm | 4.2 | Y | ||
+ | LEVOFLOXACIN | Santen | 5.6 | Y | |
+ | UNOPROSTONE ISOPROPYL | Ciba Vision | 5.6 | Y | |
| BIMATOPOST | Allergan | 5.9 | Y | |
+ | ARSENIC TRIOXIDE | Cell Therap | 5.9 | Y | |
+ | LINEZOLID (TABLET) | Pharmacia and Upjohn | 6.0 | Y | |
+ | LINEZOLID (ORAL SUSPENSION) | Pharmacia and Upjohn | 6.0 | Y | |
+ | LINEZOLID (POWDER FOR INJECTION SOLUTION) | Pharmacia and Upjohn | 6.0 | Y | |
| VALGANCICLOVIR HYDROCHLORIDE | Syntex (USA) LLC | 6.0 | Y | |
| CASPOFUNGIN ACETATE | Merck Res | 6.0 | Y | |
| OSELTAMIVIR PHOSPHATE | Roche | 6.0 | Y | |
+ | GEMTUZUMAB OZOGOMICIN | Wyeth Ayerst Labs | 6.6 | Y** | |
+ | BEXAROTENE | Ligand | 6.6 | FDA First Action: 6.0 (AE) Sponsor Response: 0.0 FDA Second Action: 0.6 (AP) | Y Y |
| TRAVOPOST | Alcon Universal | 8.3 | FDA First Action: 5.5 (AE) Sponsor Response: 0.2 FDA Second Action: 2.6 (AP) | Y Y | |
| DIDANOSINE | Bristol Myers Squibb | 9.0 | Y | ||
| ALEMTUZUMAB (BLA) | Millenium and ILEX Partners, LP | 16.5 | FDA First Action: 6.0 (RL) Sponsor Response: 1.9 FDA Second Action: 6.0 (RL) Sponsor Response: 1.0 FDA Third Action 1.6 (AP) | Y Y Y | |
| ABACAVIR SULFATE; LAMIVUDINE; ZIDOVUDINE | Glaxo Wellcome | 10.9 | FDA First Action: 5.8 (AE) Sponsor Response: 3.2 FDA Second Action: 2.0 (AP) | Y Y | |
| ZOLEDRONIC ACID | Novartis Pharms | 20.0 | FDA First Action: 9.0 (AE) Sponsor Response: 5.0 FDA Second Action: 6.0 (AP) | Y** Y | |
Table 2
FY 2000 Standard NDA and BLA Submissions Approved in FY 00 (+) and FY 01
| Generic Name | Sponsor | Approval Time (Months) | Review Goal Met | ||
|---|---|---|---|---|---|
| Total Time | Resubmissions (if necessary) | ||||
| BRIMONIDINE TARTRATE | Allergan | 8.5 | Y | ||
+ | CETRORELIX | Serono Inc | 9.4 | Y | |
| TOLTERODINE TARTRATE | Pharmacia and Upjohn | 9.8 | Y | ||
| OXCARBAZEPINE | Novartis Pharms (US) | 9.8 | Y | ||
+ | SIROLIMUS | Wyeth Ayerst Res | 9.9 | Y | |
+ | CHORIOGONADOTROPIN ALFA | Serono Labs | 9.9 | Y | |
+ | ESTRADIOL | Novartis Pharms | 9.9 | Y | |
| GRANISETRON HYDROCHLORIDE | Roche | 9.9 | Y | ||
| LEVOTHYROXINE SODIUM | Jones Pharma | 9.9 | Y | ||
| FLUVASTATIN SODIUM | Novartis Pharma | 9.9 | Y | ||
| GABAPENTIN | Parke Davis | 9.9 | Y | ||
| + | SODIUM PHOSPHATE, DIBASIC, ANHYDROUS; SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE | Inkine | 10.0 | Y | |
| + | IBUPROFEN ; PSEUDOEPHEDRINE HYDROCHLORIDE | Mcneil Cons | 10.0 | Y | |
| + | DIVALPROEX SODIUM | Abbott Labs | 10.0 | Y | |
| + | LEVOTHYROXINE SODIUM | Jerome Stevens | 10.0 | Y | |
| LANSOPRAZOLE | Tap Pharm | 10.0 | Y | ||
| ZOLMITRIPTAN | AstraZeneca Pharms | 10.0 | Y | ||
| MICONAZOLE NITRATE 2%; MICONAZOLE NITRATE 4% | Personal Prods | 10.0 | Y | ||
| BENZOYL PEROXIDE; ERYTHROMYCIN | Dermik Labs | 10.0 | Y | ||
| CLINDAMYCIN PHOSPHATE | Target Res | 10.0 | Y | ||
| DOXYCYCLINE HYCLATE | Collagenex Pharms | 10.0 | Y | ||
| INFUVITE PEDIATRIC (MULTIPLE VITAMINS) | Sabex | 10.1 | Y | ||
| CEFUROXIME SODIUM | B Braun | 10.1 | Y | ||
| LEVONORGESTREL | Berlex Labs | 10.2 | Y | ||
| TELMISARTAN; HYDROCHLOROTHIAZIDE | Boehringer Ingelheim | 10.7 | FDA First Action: 10.0 (AE) Sponsor Response: 0.3 FDA Second Action: 0.4 (AP) | Y Y | |
| METFORMIN HYDROCHLORIDE | Bristol Myers Squibb | 11.0 | Y | ||
| VALSARTAN | Novartis Pharms | 11.3 | FDA First Action: 10.0 (AE) Sponsor Response: 0.4 FDA Second Action: 1.0 (AP) | Y Y | |
| FLUOXETINE HYDROCHLORIDE | Lilly | 11.5 | FDA First Response: 9.9 (AE) Sponsor Response: 0.3 FDA Second Action: 1.3 (AP) | Y Y | |
| FLUOROURACIL | Dermik Labs | 12.0 | Y | ||
| METHYLPHENIDATE HYDROCHLORIDE | Celltech Pharms | 12.0 | FDA First Action: 10.0 (AE) Sponsor Response: 0.4 FDA Second Action: 1.6 (AP) | Y Y | |
| FORMOTEROL FUMARATE | Novartis Pharms | 12.0 | Y | ||
| TRIPTORELIN PAMOATE | Debio Recherche | 12.0 | Y | ||
| MINOCYCLINE HYDROCHLORIDE | Orapharma | 12.0 | Y | ||
| NATEGLINIDE | Novartis Pharms | 12.2 | N | ||
| MIRTAZAPINE | Organon Inc | 12.5 | FDA First Action: 10.0 (AE) Sponsor Response: 0.5 FDA Second Action: 1.9 (AP) | Y Y | |
| PEGINTERFERON ALFA-2B (BLA) | Schering Corporation | 12.9 | FDA First Action: 10.7 (RL) Sponsor Response: 0.5 FDA Second Action: 1.7 (AP) | Y1 Y | |
| LOPERAMIDE HYDROCHLORIDE; SIMETHICONE | Mcneil Cons | 13.0 | Y ** | ||
| ESOMEPRAZOLE MAGNESIUM | AstraZeneca | 14.6 | FDA First Action: 10.0 (AE) Sponsor Response: 0.5 FDA Second Action: 1.9 (AE) Sponsor Response: 0.2 FDA Second Action: 2.0 (AP) | Y Y Y | |
| ALMOTRIPTAN MALATE | Pharmacia and Upjohn | 16.6 | FDA First Action: 12.0 (AE) Sponsor Response: 2.5 FDA Second Action: 2.0 (AP) | Y Y | |
| GALANTAMINE HYDROBROMIDE (ORAL SOLUTION) | Janssen | 16.6 | FDA First Action: 9.9 (AE) Sponsor Response: 4.8 FDA Second Action: 1.8 (AP) | Y Y | |
| ACETAMINOPHEN; CLEMASTINE FUMARATE; PSEUDOPHEDRINE HYDROCHLORIDE | Novartis Cons | 16.8 | FDA First Action: 9.9 (AE) Sponsor Response: 1.2 FDA Second Action: 5.7 (AP) | Y Y | |
| ATROPINE SULFATE | Abbott Labs | 18.6 | FDA First Action: 12.0 (AE) Sponsor Response: 3.5 FDA Second Action: 3.2 (AP) | Y N | |
| CETIRIZINE HYDROCHLORIDE; PSEUDOEPHEDRINE HYDROCHLORIDE | Pfizer | 18.7 | FDA First Action: 12.0 (AE) Sponsor Response: 0.9 FDA Second Action: 5.9 (AP) | Y Y | |
| CEFDITOREN PIVOXIL | Tap Pharm | 20.0 | FDA First Action: 10.0 (AE) Sponsor Response: 4.1 FDA Second Action: 5.9 (AP) | Y Y | |
| DARBEPOETIN ALFA (BLA) | Amgen, Inc. | 20.6 | FDA First Action: 13.6 (RL) Sponsor Response: 3.0 FDA Second Action: 4.0 (AP) | Y** Y | |
| FENOFIBRATE | Abbott Labs | 21.8 | FDA First Action: 10.0 (AE) Sponsor Response: 5.7 FDA Second Action: 6.0 (AP) | Y Y | |
1CBER put out a Federal Register notice in June 2000 stating that it would not accept licensing submissions while its new tracking system was being installed. Due dates of any pending applications were extended for the duration of this moratorium.
Table 3
FY 1999 Standard NDA and BLA Submissions Approved in FY 01
| Generic Name | Sponsor | Approval Time (Months) | Review Goal Met | ||
|---|---|---|---|---|---|
| Total Time | Resubmissions (if necessary) | ||||
| BUSPIRONE HYDROCHLORIDE | Bristol Myers Squibb | 14.9 | FDA First Action: 10.0 (AE) Sponsor Response: 3.0 FDA Second Action: 1.9 (AP) | Y Y | |
| TACROLIMUS | Fujisawa Hlthcare | 15.0 | Y** | ||
| IRON SUCROSE | Luitpold Pharms | 15.0 | Y** | ||
| GALANTAMINE HYDROBROMIDE (TABLET) | Janssen Res Fdn | 17.0 | FDA First Action: 10.0 (AE) Sponsor Response: 1.1 FDA Second Action: 6.0 (AP) | Y Y | |
| DESOGESTREL; ETHINYL ESTRADIOL | Organon Inc | 19.5 | FDA First Action: 10.0 (AE) Sponsor Response: 7.5 FDA Second Action: 2.0 (AP) | Y Y | |
| CALCIUM ACETATE | Braintree Labs | 22.0 | FDA First Action: 10.0 (AE) Sponsor Response: 6.0 FDA Second Action: 6.0 (AP) | Y Y | |
| CHLORHEXIDINE GLUCONATE; ETHYL ALCOHOL | 3M Hlth (US) | 23.4 | FDA First Action: 12.0 (NA) Sponsor Response: 8.1 FDA Second Action: 3.4 (AP) | Y Y | |
| ACETAMINOPHEN ; TRAMADOL HYDROCHLORIDE | RW Johnson | 23.5 | FDA First Action: 10.0 (AE) Sponsor Response: 4.5 FDA Second Action: 6.0 (AE) Sponsor Response: 1.0 FDA Third Action: 2.0 (AP) | Y Y Y | |
| BOTULINUM TOXIN TYPE B (BLA) | Elan Pharmaceuticals | 23.6 | FDA First Action: 10.0 (RL) Sponsor Response: 4.7 FDA Second Action: 6.7 (RL) Sponsor Response: 0.2 FDA Third Action: 2.0 (AP) | Y Ya Y | |
| DICLOFENAC SODIUM | Bioglan Pharma PLC | 23.8 | FDA First Action: 12.0 (NA) Sponsor Response: 3.1 FDA Second Response 5.8 (AE) Sponsor Response: 0.9 FDA Third Action: 2.0 (AP) | Y Y Y | |
| DROSPIRENONE ; ETHINYL ESTRADIOL | Berlex Labs | 23.8 | FDA First Action: 10.0 (AE) Sponsor Response: 1.8 FDA Second Action: 2.0 (AE) Sponsor Response: 4.1 FDA Third Action: 5.9 (AP) | Y Y Y | |
| SULFAMETHOXAZOLE; TRIMETHOPRIM; PHENAZOPYRIDINE HYDROCHLORIDE | Able Labs | 24.7 | FDA First Action: 15.0 (NA) Sponsor Response: 8.1 FDA Second Action: 1.6 (AP) | Y** Y | |
| DIGOXIN IMMUNE FAB (OVINE) (BLA) | Protherics Inc. | 24.9 | FDA First Action: 13.6 (RL) Sponsor Response: 5.3 FDA Second Action: 6.0 (AP) | Y** Y | |
| HEPATITIS A INACTIVATED & HEPATITIS B (RECOMBINANT) VACCINE (BLA) | SmithKline Beecham Biologicals | 27.3 | FDA First Action: 9.9 (RL) Sponsor Response: 2.0 FDA Second Action: 5.8 (RL) Sponsor Response: 3.5 FDA Third Response 6.0 (AP) | Y Y Y | |
| PERFLUTREN | Dupont Pharms | 31.7 | FDA First Action: 10.0 (AE) Sponsor Response: 4.0 FDA Second Action: 5.9 (AE) Sponsor Response: 5.9 FDA Third Response: 6.0 (AP) | Y Y Y | |
Table 4
FY 1998 Standard NDA and BLA Submissions Approved in FY 01
| Generic Name | Sponsor | Approval Time (Months) | Review Goal Met | ||
|---|---|---|---|---|---|
| Total Time | Resubmissions (if necessary) | ||||
| BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE | Dermik Labs | 17.42 | FDA First Action: 11.7 (NA) Sponsor Response: 15.0 FDA Second Action: 5.7 (AP) | Y Y | |
| NESIRITIDE | Scios | 19.03 | FDA First Action: 12.0 (NA) Sponsor Response: 20.5 FDA Second Action: 5.8 (AE) Sponsor Response: 0.9 FDA Third Action: 0.3 (AP) | Y Y Y | |
| ALBUTEROL SULFATE (INHALATION AEROSOL) | Glaxo | 21.54 | FDA First Action: 12.0 (AE) Sponsor Response: 12.1 FDA Second Action: 6.0 (AE) Sponsor Response: 0.1 FDA Third Action: 3.4 (AP) | Y Y Y | |
| AMOXICILLIN; CLAVULANATE POTASSIUM | GlaxoSmithKline | 26.35 | FDA First Action: 11.8 (AE) Sponsor Response: 17.4 FDA Second Action: 6.0 (AE) Sponsor Response: 2.6 FDA Third Action: 6.0 (AP) | Y Y Y | |
| CROTALIDAE POLYVALENT IMMUNE FAB (OVINE) (BLA) | Protherics Inc | 29.1 | FDA First Action: 12.0 (RL) Sponsor Response: 2.6 FDA Second Action: 6.0 (RL) Sponsor Response: 3.2 FDA Third Action: 5.4 (AP) | Y Y Y | |
| CALCIUM CARBONATE; FAMOTIDINE; MAGNESIUM HYDROXIDE | Merck Res | 31.9 | FDA First Action: 12.0 (NA) Sponsor Response: 10.0 FDA Second Action: 6.0 (AE) Sponsor Response: 1.4 FDA Third Action: 2.4 (AP) | Y Y Y | |
| PANTOPRAZOLE SODIUM | Wyeth Ayerst Labs | 32.1 | FDA First Action: 12.0 (AE) Sponsor Response: 1.4 FDA Second Action: 5.8 (AE) Sponsor Response: 2.2 FDA Third Action: 6.0 (AE) Sponsor Response: 2.7 FDA Fourth Action: 1.9 (AP) | Y Y Y Y | |
| ALBUTEROL SULFATE; IPRATROPIUM BROMIDE | Dey Labs | 33.8 | FDA First Action: 12.0 (AE) Sponsor Response: 6.2 FDA Second Action: 6.0 (AE) Sponsor Response: 3.6 FDA Third Action: 6.0 (AP) | Y Y Y | |
| BIVALIRUDIN | The Medicines Company | 35.8 | FDA First Action: 10.8 (NA) Sponsor Response: 5.3 FDA Second Action: 6.0 (AE) Sponsor Response: 0.5 FDA Third Action: 6.0 (AE) Sponsor Response: 2.2 FDA Fourth Action: 5.0 (AP) | Y Y Y Y | |
| ALBUTEROL SULFATE (INHALATION SOLUTION) | Dey Labs | 37.1 | FDA First Action: 12.0 (AE) Sponsor Response: 8.3 FDA Second Action: 6.0 (AE) Sponsor Response: 4.8 FDA Third Action: 6.0 (AP) | Y Y Y | |
2The total approval time for this NDA was adjusted because a manufacturing facility was not available. The time period from 4/1/99 to 6/30/00 was excluded while the facility was being rebuilt.
3 The time period from 4/27/99 to 1/10/01 was excluded from the total approval time while the sponsor designed and conducted new clinical trials.
4 The time period from 7/1/99 to 7/3/00 was excluded from the total approval time while the sponsor submitted new manufacturing and methods needed for approval.
5The time period from 10/26/98 to 4/6/00 was excluded from the total approval time. The sponsor submitted new clinical data to support the indication.
Table 5
FY 1997 Standard NDA and BLA Submissions Approved in FY 01
| Generic Name | Sponsor | Approval Time (Months) | Review Goal Met | ||
|---|---|---|---|---|---|
| Total Time | Resubmissions (if necessary) | ||||
| ESTRADIOL CYPIONATE; MEDROXYPROGESTERONE | Pharmacia and Upjohn | 24.06 | FDA First Action: 12.0 (NA) Sponsor Response: 6.7 FDA Second Action: 6.0 (AE) Sponsor Response: 5.8 FDA Third Action: 6.0 (AP) | Y Y Y | |
| FORMOTEROL FUMARATE | Novartis Pharms | 24.07 | FDA First Action: 12.0 (AE) Sponsor Response: 17.0 FDA Second Action: 6.0 (AE) Sponsor Response: 2.8 FDA Third Action: 6.0 (AP) | Y Y Y | |
| ZIPRASIDONE HYDROCHLORIDE | Pfizer Cent Res | 46.7 | FDA First Action: 15.0 (NA) Sponsor Response: 20.8 FDA Second Action: 6.0 (AE) Sponsor Response: 1.5 FDA Third Action: 3.5 (AP) | Y Y Y | |
6The total approval time for this NDA was adjusted. The application did not contain adequate data collection for safety and efficacy assessments. The time period from 9/25/98 to 4/14/99 was excluded. FDA also requested further clinical trials to determine added benefits of this combination product. The time period from 10/15/99 to 4/7/00 was excluded.
7The total approval time for this NDA was adjusted because the application did not contain adequate stability data meeting agency standards. A follow-up stability submission did not address all the packaging and storage conditions. The time periods from 6/26/98 to 11/24/99 and 5/24/00 to 8/18/00 were excluded while new stability studies were being performed.







