Metal Hydrides
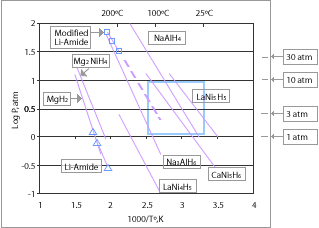
Metal hydrides have the potential for reversible on-board hydrogen storage and release at low temperatures and pressures. The optimum "operating P-T window" for PEM fuel cell vehicular applications is in the range of 1-10 atm and 25-120°C. This is based on using the waste heat from the fuel cell to "release" the hydrogen from the media. In the near-term, waste heat less than 80°C is available but as high temperature membranes are developed, there is potential for waste heat at higher temperatures. A simple metal hydride such as LaNi5H6, that incorporates hydrogen into its crystal structure, can function in this range, but its gravimetric capacity is too low (~1.3 wt.%) and its cost is too high for vehicular applications.
Complex metal hydrides such as alanate (AlH4) materials have the potential for higher gravimetric hydrogen capacities in the operational window than simple metal hydrides. Alanates can store and release hydrogen reversibly when catalyzed with titanium dopants, according to the following 2-step displacive reaction for sodium alanate:
NaAlH4 = 1/3 Na3AlH6 +2/3Al+H2
Na3AlH6 = 3 NaH + Al + 3/2H2
At 1 atm pressure, the first reaction becomes thermodynamically favorable at temperatures above 33°C and can release 3.7 wt.% hydrogen, while the second reaction takes place above 110°C and can release 1.8 wt.% hydrogen. The amount of hydrogen that a material can release, rather than only the amount the material can hold, is the key parameter used to determine system (net) gravimetric and volumetric capacities.
Issues with complex metal hydrides include low hydrogen capacity, slow uptake and release kinetics, and cost. The maximum material (not system) gravimetric capacity of 5.5 wt.% hydrogen for sodium alanate is below the 2010 DOE system target of 6 wt.%. Thus far, 4 wt.% reversible hydrogen content has been experimentally demonstrated with alanate materials. Also, hydrogen release kinetics are too slow for vehicular applications. Furthermore, the packing density of these powders is low (for example roughly 50%) and the system-level volumetric capacity is a challenge. Although sodium alanates will not meet the 2010 targets, it is envisioned that their continued study will lead to fundamental understanding that can be applied to the design and development of improved types of complex metal hydrides.
Recently, a new complex hydride system based on lithium amide has been developed. For this system, the following reversible displacive reaction takes place at 285°C and 1 atm:
Li2NH + H2 = LiNH2 + LiH
In this reaction, 6.5 wt.% hydrogen can be reversibly stored, with potential for 10 wt.%. However, the current operating temperature is outside of the vehicular operating window. However, the temperature of this reaction can be lowered to 220°C with magnesium substitution, although at higher pressures. Further research on this system may lead to additional improvements in operating conditions with improved capacity.
One of the major issues with complex metal hydride materials, due to the reaction enthalpies involved, is thermal management during refueling. Depending on the amount of hydrogen stored and refueling times required, megawatts to half a gigawatt must be handled during recharging on-board vehicular systems with metal hydrides. Reversibility of these and new materials also needs to be demonstrated for over a thousand cycles.
 |
Learn about DOE's Metal Hydride Storage R&D Activities.




















