Advertisement
Advertise Here
-
January 10, 2011 - Volume 89, Number 2
- p. 4
Specialties: Transaction will create a leader in water treatment and cleaning products.
Mitsui & Co., Dow Chemical joint venture in Brazil will be based on sugarcane.
Biogeochemistry: Acidifying phosphorous-containing dust makes element more accessible to ocean ecosystems.
Climate Change: By adding a treatment step, plants handling wastewater could cut N2O emissions.
Energy Policy: Clean power from livestock waste would become economical with carbon tax.
Chemical Defense: Proteomic analysis of tree frog secretions reveals both salves and weapons akin to snake venom.
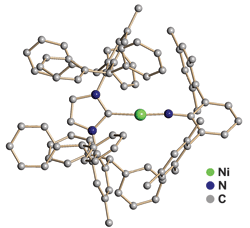
A multi-institution research team has prepared the first two-coordinate transition-metal complex containing an imido ligand. This coordinatively unsaturated nickel(II) complex demonstrates enhanced chemical reactivity over more highly coordinated complexes and potentially signals a new facet of research for organometallic chemists.
The active versions of transition-metal complexes in many chemical reactions, such as olefin metathesis and the reduction of nitrogen to ammonia, contain multiple bonds between a metal with a vacant d orbital and a ligand with abundant electron density. But chemists struggle to re-create these reactive intermediates as isolable molecules.
In a new study, Carl A. Laskowski and Gregory L. Hillhouse of the University of Chicago and coworkers show one way to do it: They pair nickel with an extremely bulky imidazole N-heterocyclic carbene ligand on one side and a bulky terphenyl imido ligand on the other (J. Am. Chem. Soc., DOI: 10.1021/ja1101213).
The “massive steric profile” of the imidazole helps prevent more than two ligands from accessing the nickel, Hillhouse notes. As a result, the complex adjusts its electronic structure by forming a linear C–Ni=N core dominated by π-bonding. The nickel atom features a “high spin” d8 electron configuration with two unpaired electrons, whereas most two-coordinate transition-metal complexes have a paired d10 electron configuration. Study coauthor Thomas R. Cundari of the University of North Texas confirmed these experimental findings with density functional theory calculations.
Reactive substrates such as carbon monoxide and ethylene readily add across the Ni=N bond, resulting in the transfer of the imido group to the substrate to form isocyanates and vinylamines, respectively. This reactivity is greatly enhanced relative to three-coordinate nickel imido complexes, Hillhouse says.
“This piece of work illustrates a new way to effect the catalytic synthesis of organonitrogen compounds,” says Christopher C. Cummins of Massachusetts Institute of Technology, whose group focuses on using large ligands to make low-coordinate complexes. “The two-coordinate nickel imido catalyst itself represents a breakthrough in the chemistry of nickel,” Cummins adds, “and the simplicity of the transfer reaction system renders it amenable to a high degree of mechanistic analysis.”
ACS is the leading employment source for recruiting scientific professionals. ACS Careers and C&EN Classifieds provide employers direct access to scientific talent both in print and online. Jobseekers | Employers
Join more than 161,000 professionals in the chemical sciences world-wide, as a member of the American Chemical Society.
» Join Now!