Final Report: Nearcritical Water as a Reaction Solvent
EPA Grant Number: R828130Title: Nearcritical Water as a Reaction Solvent
Investigators: Eckert, Charles A. , Liotta, C. L.
Institution: Georgia Institute of Technology
EPA Project Officer: Karn, Barbara
Project Period: June 1, 2000 through June 30, 2003
Project Amount: $397,910
RFA: Technology for a Sustainable Environment (1999)
Research Category: Pollution Prevention/Sustainable Development
Description:
Objective:The objective of this research project was to explore the use of nearcritical water (NCW) as a solvent for industrial chemical reactions, as well as characterize the solvent for the purposes of reactor design and scaleup. This work demonstrated that NCW processes may be developed with the potential to replace environmentally undesirable solvents and eliminate many hundreds of millions of tons of waste per year. These processes not only will be environmentally superior, but also will offer improved economics for increased competitiveness. In the course of this project we examined:
· NCW as a benign replacement solvent for harmful organic chemicals.
·
A wide range of synthetic opportunities by acid and base catalysis.
·
Carbon-carbon bond formation.
·
Driving reactions to higher conversion.
·
Hydrolysis and other condensation reactions.
·
Utilization of unique phase behavior to achieve difficult separations.
·
Phase equilibria or organics and water at high temperature.
·
Potential for sustainable industrial-scale applications.
The specific goals of this project focused on reaction kinetics and equilibria, and phase equilibria in high-temperature water for pollution prevention and the development of sustainable industrial processes. This work also impacts a much broader scientific area. The phase equilibria of organic/water systems are necessary measurements for refining, tertiary oil recovery, and geology. On an even more profound level, the conditions for reactions in NCW are similar to the environment of geothermal vents of the floor of the deep ocean. The unusual chemistry, biochemistry, and biology of these deep ocean vents may be representative of the earth’s environment millions of years ago and are of considerable interest to scientists who study the origin(s) of life.
Summary/Accomplishments (Outputs/Outcomes):This project provides the basis for adapting a variety of chemical processes to NCW as that benign solvent. NCW offers significant engineering advantages. It dissolves both organics and salts, and thus makes normally heterogeneous reactions homogeneous. It acts as a natural acid and base, orders of magnitude stronger than ambient water, so no added acids (or bases) are required for many reactions, and there is no subsequent need for neutralization and salt disposal. Moreover, in most typical reaction processes, the separation accounts for 60-80 percent of the capital and operating costs. With organic reactions in NCW, the separation can be as simple as mere cooling and decanting.
The solubilities of several organic compounds (toluene, acetophenone, 1-octanol,
anisole) in water were measured up to their upper critical solution temperatures
(220-280°C). The Kamlet-Taft solvent parameters  *,
*,  , and
, and  of saturated
liquid water have been determined from 25-275°C based on solvatochromic
measurements and show that the polarity and hydrogen-bonding of water are highly
tunable properties with temperature. These pieces of information can be used
together to model the solution behavior of these organics in water at high
temperature.
of saturated
liquid water have been determined from 25-275°C based on solvatochromic
measurements and show that the polarity and hydrogen-bonding of water are highly
tunable properties with temperature. These pieces of information can be used
together to model the solution behavior of these organics in water at high
temperature.
Additionally, we also have used the hydrolyses of several substituted benzoate esters and a series of substituted anisoles as probes to elucidate the activity of the two ionic species in NCW. Each of these hydrolyses can run via both acid- and base-catalyzed pathways, as well as an SN2 pathway. Analysis of Hammett plots suggests that the benzoate esters hydrolyze autocatalytically, following an acid-catalyzed mechanism, while the anisoles are hydrolyzed via the SN2 mechanistic pathway. This work suggests that NCW offers significant potential, both as a benign solvent and as a reaction medium that does not require neutralization with acid or base after the desired reaction is complete. This work examined the acid catalyzing potential of NCW.
We also explored the carbon-carbon bond formation in NCW. The Claisen-Schmidt condensation of benzaldehyde with 2-butanone demonstrated the ability of NCW to conduct conventionally acid- or base-catalyzed reactions homogeneously without the addition of a catalyst.
The temperature-dependent thermodynamics and kinetics of the hydrolyses of 4-nitroaniline and N,N-dimethyl-4-nitroaniline were investigated to demonstrate the effectiveness of Kamlet-Taft linear solvation energy relationship (LSER) and the ET(30) parameter for correlating kinetic data in NCW. These methods may provide a tool to describe other chemical reactions in this novel medium.
Investigations into the Friedel-Crafts acylation of isobutylbenzene and trans-styrylacetic acid were performed over a temperature range of 250-350°C. The acylation of isobutylbenzene with acetic acid produced the desired product, but at low yields as a result of the equilibrium limitations associated with the large concentrations of water present in this system. Investigations into the acylation of trans-styrylacetic acid also were successful and were not equilibrium-limited; however, they were hampered by the decomposition of the starting material into undesirable products.
We used two experimental techniques for measuring 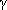
 in NCW—headspace
and dynamic liquid chromatography. Headspace chromatography involves analysis
of the gas (vapor) phase of a binary heterogeneous system in equilibrium with
a very dilute liquid. Our dynamic chromatography measured
in NCW—headspace
and dynamic liquid chromatography. Headspace chromatography involves analysis
of the gas (vapor) phase of a binary heterogeneous system in equilibrium with
a very dilute liquid. Our dynamic chromatography measured 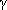
 of organic
compounds in NCW as a function of temperature, using a novel column developed
by Carr, capable of withstanding the temperature and chemical activity of water
to elevated temperatures. We determined
of organic
compounds in NCW as a function of temperature, using a novel column developed
by Carr, capable of withstanding the temperature and chemical activity of water
to elevated temperatures. We determined 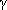
 in NCW of several homologous
series of organic compounds.
in NCW of several homologous
series of organic compounds.
We also examined the solvatochromic parameters of ethanol in the liquid and nearcritical regions (25-225°C). A comparison of these values with NCW provides key insight into the similarities and differences of the two media, and highlighted the potential of each for benign organic synthesis.
In addition to completing the project objective of exploring water as a reaction solvent, this project resulted in 10 publications and numerous presentations. It supported the research activities of a total of nine undergraduate and Ph.D. students, and two research scientists. Seven of these have completed their studies during this project:
James Brown, Ph.D., now a Research Coordinator at Georgia Technological Institute
Kris Griffith, Ph.D., now a Research Scientist at American Pacific Corporation
Jason Hallett, Ph.D., now a Postdoctoral Fellow at Georgia Technological Institute
Heather Lesutis, Ph.D., now a Lecturer at Oxford College of Emory University
Shane Nolen, Ph.D., now a Lithography Specialist at Intel Corporation
Griffin Smith, B.S., now a Ph.D. candidate at the University of Texas
Kevin West, Ph.D., now a Postdoctoral Research Associate at the University
of Minnesota.
Considerable technical challenges exist for the engineering of chemical manufacturing with environmentally benign solvents. There are very considerable environmental advantages to our society in replacing less desirable organic chemical solvents with water. This project demonstrated the technical feasibility of NCW for the advancement and implementation of sustainable technology.
NCW provides environmentally benign chemical processing alternatives to the chemical and pharmaceutical industries by replacing hazardous solvents and reducing waste. We have demonstrated water's ability to replace undesirable solvents and eliminate the need for added catalysts in a variety of chemical reactions, including Friedel-Crafts acylations. In these reactions, NCW solubilizes the organics and acts as a source of both hydronium and hydroxide ions, thereby replacing the normally required hazardous solvents and catalysts that require subsequent neutralization and disposal. We measured phase behavior and model reactions in NCW and determined that it has an excellent potential as an environmentally benign replacement solvent for a wide variety of chemical reactions. Example reactions demonstrated include: hydrolyses, condensations, alkylations, and acylations.
Journal Articles on this Report : 11 Displayed | Download in RIS Format
| Other project views: | All 34 publications | 11 publications in selected types | All 11 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Brown JS, Glaser R, Liotta CL, Eckert CA. Acylation of activated aromatics without added acid catalyst. Chemical Communications 2000;14:1295-1296. |
R828130 (Final) |
not available |
|
|
Brown JS, Hallett JP, Bush D, Eckert CA. Liquid-liquid equilibria for binary mixtures of water plus acetophenone, plus 1-octanol, plus anisole, and plus toluene from 370 K to 550 K. Journal of Chemical and Engineering Data 2000;45(5):846-850 |
R828130 (Final) |
not available |
|
|
Eckert CA, Liotta CL, Brown JS. Clean solution for chemical synthesis. Chemistry and Industry 2000;3:94-97. |
R828130 (Final) |
not available |
|
|
Eckert CA, Bush D, Brown JS, Liotta CL. Tuning solvents for sustainable technology. Industrial and Engineering Chemistry Research 2000;39(12):4615-4621. |
R828130 (Final) |
not available |
|
|
Jones JR, Liotta CL, Collard DM, Schiraldi DA. Photochemical cross-linking of poly(ethylene terephthalate-co-2,6-anthracenedicarboxylate). Macromolecules 2000;33(5):1640-1645 |
R828130 (Final) |
not available |
|
|
Lesutis HP, Glaser R, Liotta CL, Eckert CA. Acid/base-catalyzed ester hydrolysis in near-critical water. Chemical Communications 1999;(20):2063-2064 |
R828130 (Final) R825325 (Final) |
not available |
|
|
Patrick HR, Griffith K, Liotta CL, Eckert CA, Glaser R. Near-critical water: A benign medium for catalytic reactions. Industrial & Engineering Chemistry Research 2001;40(26):6063-6067 |
R828130 (Final) |
not available |
|
|
Lu J, Brown JS, Liotta CL, Eckert CA. Polarity and hydrogen-bonding of ambient to near-critical water: Kamlet-Taft solvent parameters. Chemical Communications 2001;(7):665-666 |
R828130 (Final) |
not available |
|
|
Lu J, Boughner EC, Liotta CL, Eckert CA. Nearcritical and supercritical ethanol as a benign solvent: polarity and hydrogen-bonding. Fluid Phase Equilibria 2002;198(1):37-49. |
R828130 (Final) |
not available |
|
|
Lu J, Brown JS, Boughner EC, Liotta CL, Eckert CA. Solvatochromic characterization of near-critical water as a benign reaction medium. Industrial & Engineering Chemistry Research 2002;41(12):2835-2841 |
R828130 (Final) |
not available |
|
|
Nolen SA, Liotta CL, Eckert CA, Glaser R. The catalytic opportunities of near-critical water: a benign medium for conventionally acid and base catalyzed condensations for organic synthesis. Green Chemistry 2003;5(5):663-669 |
R828130 (Final) |
not available |
green chemistry, solvent alternatives, organics, environmental chemistry, engineering, modeling. , Sustainable Industry/Business, Scientific Discipline, RFA, Technology for Sustainable Environment, Sustainable Environment, Chemical Engineering, Environmental Engineering, Environmental Chemistry, cleaner production, green chemistry, carbon bond formation, organic chemicals, nearcritical water, environmentally benign solvents, hydroxide ions, hydrolysis, alternative materials, source reduction, reaction solvent, carbon carbon bond, innovative technology
Progress and Final Reports:
Original Abstract
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20090825064951im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)