2002 Progress Report: Dry Lithography: Environmentally Responsible Processes for High Resolution Pattern Transfer and Elimination of Image Collapse using Positive Tone Resists
EPA Grant Number: R829586Title: Dry Lithography: Environmentally Responsible Processes for High Resolution Pattern Transfer and Elimination of Image Collapse using Positive Tone Resists
Investigators: DeSimone, Joseph M.
Institution: University of North Carolina at Chapel Hill
EPA Project Officer: Richards, April
Project Period: November 1, 2001 through November 1, 2004
Project Period Covered by this Report: November 1, 2001 through November 1, 2002
Project Amount: $347,898
RFA: Technology for a Sustainable Environment (2001)
Research Category: Pollution Prevention/Sustainable Development
Description:
Objective:Conventional lithography (the means by which integrated circuits and memory devices are produced) requires over 1 kg of organic solvent and aqueous waste to produce a 2-g chip. Carbon dioxide can be utilized as an environmentally responsible alternative for aqueous and organic solvents in a variety of processes. Previous research in our laboratory has shown the utility of CO2 in the lithographic process at 193 nm. However, industry continues to use smaller wavelengths to decrease feature size and increase performance, with the next lithographic wavelength being 157 nm. The objective of this research project is to capitalize on our success with 193-nm negative tone images in CO2 by developing a 157-nm positive tone image for a CO2 based lithographic system.
In addition to the environmental benefits of switching from aqueous and organic solvents to CO2, dramatic performance benefits also should be realized. Industry is using larger wafers, which must be spin coated with a photoresist. By utilizing CO2, larger surfaces can be coated at lower spin rates and concentrations. Furthermore, during the development of small images (< 70 nm) with aqueous bases, the potential for image collapse is very high given the high-surface tension of water. By replacing water with liquid or supercritical CO2 and a low-surface tension fluid, the potential for image collapse should be eliminated.
Progress Summary:The development of a 157-nm positive tone image requires the deposition, exposure, development, etching, and stripping of a 157-nm photoresist. A positive tone image is obtained when the exposed region changes solubility such that on subsequent development, the exposed regions are removed. To adapt this system to CO2, a 157-nm photoresist must be made that possesses liquid CO2 solubility for deposition, a method for making the material more soluble in CO2 upon exposure to 157-nm light, and finally stripping of the unexposed resist after etching. Other criteria include the necessity for low absorbance at 157 nm to allow for complete latent image transfer, relatively high-glass transition temperature to prevent image blurring, and etch resistance that will allow image transfer to the substrate.
In the past year, we focused on 157-nm photoresist development following two thrusts. The first thrust involves the development of a solubility contrast that can be utilized in CO2. The second thrust centers on the synthesis of a highly CO2 soluble and optically transparent (at 157 nm) backbone to which the contrast can be attached.
CO2 is a great solvent for nonpolar fluorinated compounds. So, the design of the photoresist requires that the material be somewhat polar prior to exposure and less polar after exposure. Therefore, the chemical reaction that occurs during exposure must mimic this polar to less-polar switch. As such, we have targeted the Pinacol rearrangement reaction (see Figure 1 below) as the basis for our solubility contrast.
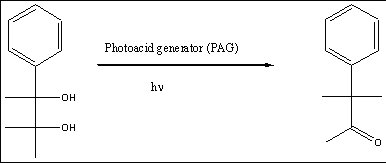
Figure 1. The Pinacol Rearrangement Reaction.
In the past year, we have prepared the precursor shown in Figure 1 as a model to study the rearrangement. Furthermore, we have prepared several polymers (see Figure 2), which possess CO2 solubility so that upon exposure to acid and subsequent rearrangement, the solubility change can be examined.

Figure 2. Polymers That Possess CO2 Solubility.
These materials are useful for proof of concept, but the glass transition temperature (~55°C) is too low. Furthermore, the multiple double bonds in the styrenic monomer are too strongly absorbing for 157-nm lithography. Therefore, as part of the second thrust, a backbone structure is being examined, which will possess a higher glass transition temperature and low absorbance, while maintaining effective etch resistance and CO2 solubility.

Figure 3. Materials That Possess High-Glass Transition Temperature (140°C for A and 147°C for B).
The materials shown in Figure 3 have been prepared. These materials possess relatively high-glass transition temperatures (140°C for A and 127°C for B). Absorbances at 157 nm are 1.7 and 3.1 mm-1 for A and B, respectively. Given the fairly high absorbance of B, this material will not be examined further. Etch resistance of A is promising, with the material etching only 20 percent faster than the industry standard Novolak. Unfortunately, neither material is liquid CO2 soluble.
In conclusion, we have prepared model compounds to prove our CO2 solubility contrast concept. We also have prepared backbone structures that meet our glass transition temperature and 157-nm absorbance requirements.
Future Activities:In the near future, we will begin to test our model compounds to prove our solubility contrast concept. This will involve studying the kinetics of the rearrangement, both in the small molecule and polymer model compound. Solubility changes in CO2 will be examined on the model polymer compound. Backbone work will involve incorporating other monomers, which will maintain the low absorbance and high-glass transition temperature, while increasing CO2 solubility and improving etch resistance.
Upon completion of these two thrusts, the two components will be incorporated together to give us our 157-nm photoresist. This material will then be deposited via our CO2-based spin coater and imaged with our new ASML 5,000/900 series 193-nm stepper, which should be running by midsummer. This tool will allow immediate feedback as to conditions that are needed to produce high-pattern transfer. In conjunction with CO2 development, conditions which minimize image collapse will more readily be obtained. This will allow rapid extension of this dry lithographic system to 157 nm.
Journal Articles on this Report : 1 Displayed | Download in RIS Format
| Other project views: | All 16 publications | 9 publications in selected types | All 9 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Zannoni LA, DeSimone JM. Synthesis, characterization, and properties of copolymers prepared in dense carbon dioxide towards the development of a 157 nm Photoresist. Polymeric Materials Science and Engineering 2002;87:197-198. |
R829586 (2002) |
not available |
green chemistry, alternatives, clean technologies, innovative technology, waste reduction, environmentally conscious manufacturing, carbon dioxide, CO2, pattern transfer, image collapse, photoacid generators, fluoropolymers, spin coating. , Sustainable Industry/Business, Scientific Discipline, RFA, Technology for Sustainable Environment, Sustainable Environment, Chemical Engineering, Environmental Engineering, Environmental Chemistry, Economics and Business, supercritical carbon dioxide, cleaner production, clean technologies, green design, microelectronics, green chemistry, alternative solvents, in-process waste minimization, engineering, environmentally benign solvents, alternative materials, dry lithography, high resolution pattern transfer, environmentally conscious design, solvent substitute, supercritical carbon dioxide (SCCO2) technology, pollution prevention
Relevant Websites:
Progress and Final Reports:
Original Abstract
2003 Progress Report
Final Report
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20090825080005im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)