Final Report: Pollution Prevention with the Use of Molecular Assemblies
EPA Grant Number: R825327Title: Pollution Prevention with the Use of Molecular Assemblies
Investigators: Warner, John C.
Institution: University of Massachusetts - Boston
EPA Project Officer: Karn, Barbara
Project Period: October 15, 1996 through October 14, 1999 (Extended to December 14, 2000)
Project Amount: $344,713
RFA: Technology for a Sustainable Environment (1996)
Research Category: Pollution Prevention/Sustainable Development
Description:
Objective:In many sectors of the chemical industry a lead compound often is identified as having some behavior worthy of further exploration. To arrive at the physical and chemical properties specified by a particular application, the synthetic organic chemist will traditionally modify the lead compound by altering functional groups within the molecule's covalent framework. Structure-activity relationships are elucidated in order to determine the most effective molecular derivative for a particular application. The physical properties of the molecule (melting point, solubility, vapor pressure, diffusivity, etc.) thus are manipulated. The number of potential derivatives obtainable by this process is limited only by the synthetic chemist’s imagination and skill. After the candidate molecules have been synthesized, they then are evaluated for performance within the specific application. A typical research and development program can involve the synthesis and evaluation of hundreds of derivative candidates. The environmental toll (e.g., organic solvent use, generation of hazardous materials) and economic price (e.g., disposal costs, labor costs) of such a process is quite high. There are several new techniques designed to make this process more efficient. This grant program itself is a testament to the number of diverse and creative ways that scientists are successfully improving the environmental consequences of synthetic and manufacturing procedures. In general terms, the bulk physical properties of a given substance is a consequence of the sum of its intermolecular interactions between identical molecules and molecules of its environment. The forces of attraction and repulsion between the molecules themselves and the forces of attraction and repulsion between the molecule and the molecules in its immediate vicinity arise from interactions of functional groups.
The overall objective of this research project was to explore a different approach to the manipulation of physical and chemical properties. Non-covalent derivatization is the specific application of the principles of molecular recognition and self-assembly to control the physical and chemical properties of a molecular species in which auxiliary molecules are added to modulate the interaction of the target molecules with each other and the surrounding chemical environment. The technique of non-covalent derivatization employs non-covalent intermolecular forces (hydrogen bonding, p-stacking, lipophilic-lipophilic interactions and electrostatic interactions) to trap the molecular species in organized matrices. The procedure of forming molecular assemblies to manipulate bulk physical properties allows for reduced usage of chemical resources. For example, instead of performing several time-consuming, solvent-based, chemical reactions to synthesize a series of candidate compounds for structure activity studies, non-covalent derivatization allows for the addition of simple, inexpensive, readily available "complexing reagents." For this to be successful as pollution prevention, these assemblies must significantly reduce the number of synthetic reactions carried out. Often the formation of these assemblies involves no organic solvents because the supramolecular structures often can be constructed via solid state grinding or aqueous dispersing techniques.
By understanding the molecular topography of the simplest reactive species (the simplest derivative of the lead compound), bulk physical properties may be modified in an environmentally benign manner by non-covalent complexation with auxiliary reagents. Rather than derivatize a molecule by covalently attaching functional groups, one may be able to form a stoichiometric association with a simple conjugate molecule—thereby imparting the desired physical property.
When using non-covalent derivatization, the chemist identifies what aspects
of the lead compound can be exploited through intermolecular interactions.
Is the molecule capable of participating in a hydrogen bonding network, a  -stacking
complex, lipophilic aggregation, or coulombic packing? Based on this evaluation,
specific candidate auxiliary reagents may be determined. To achieve pollution
prevention using this technique the auxiliary complexing reagent must be environmentally
benign and relatively inexpensive.
-stacking
complex, lipophilic aggregation, or coulombic packing? Based on this evaluation,
specific candidate auxiliary reagents may be determined. To achieve pollution
prevention using this technique the auxiliary complexing reagent must be environmentally
benign and relatively inexpensive.
To understand the factors that contribute to non-covalent derivatization in solid-state structures, our research group systematically investigated the dialkyl amide-phenol hydrogen bonding in binary self-assembled systems. In these systems, the dialkyl amide functions as a hydrogen bond acceptor through the lone-pair electrons on the carbonyl oxygen. The amide has no acidic protons capable of hydrogen bond donation, making this an excellent component in the binary systems because it can only function as a hydrogen bond acceptor. The phenol acts as a hydrogen bond donor. It also has two lone pairs of electrons that can be hydrogen bond acceptors. This ability to act as an acceptor and a donor can cause competition with the amide for hydrogen bonding. The phenol can form a hydrogen bond with another phenol.

In a competition for hydrogen bonding, it is likely that the amide is a better hydrogen bond acceptor than the phenol because of the resonance form that localizes a negative charge on the oxygen. When a molecule that has more than one hydrogen bond accepting functional group forms a complex with a molecule that has more than one hydrogen bond donating group, extended hydrogen bonding throughout the solid may occur. For example, x-ray crystallographic results (Foxman et al., 1998; Bai, 1998) indicate that a 1:1 cocrystal of hydroquinone (having two hydrogen bond donating sites) and bis(N,N-diethyl)terephthalamides (having two hydrogen bond accepting functional groups) is held together by hydrogen bonds in a linear pattern through the crystal lattice. In this system, the hydroquinone and bis[N,N-dialkyl]terephthalamide form can be viewed as an alternating copolymer held together by hydrogen bonds. The chains of these “hydrogen bond polymers” in turn are held together through London forces between the alkyl side chains. When the ethyl groups are replaced with methyl groups, the competitive behavior of phenol to act as a hydrogen bond acceptor can be observed. In this case, the "hydrogen bond polymer" of alternating hydroquinone and bis[N,N-dimethyl]terephthalamide is essentially identical to that in the diethyl derivative; however, the void in space, created by the elimination of the methylene units, allows for the incorporation of a second hydroquinone molecule, forming new hydrogen bonds between consecutive hydroquinones and giving a 1:2 cocrystal with bis[N,N-dimethyl]terephthalamide and hydroquinone. These second hydroquinones can be thought of as "linking units" between the existing hydroquinone-terephthalamide chains. Alternatively, the terephthalamides can be viewed as "linking units" between the existing hydroquinone chains. A third type of structure also exists in these systems. The bis-[N,N-dimethyl]terephthalamide also forms a 1:1 complex with hydroquinone. Here, there are no extended hydrogen bonds throughout the lattice. Instead, isolated rings of tetramers define the system. These systems define three possible hydrogen bonding motifs: chains, networks, and isolated systems.

The role of aromaticity has been investigated (Foxman, et al., 1999; Tassa, 1998). The bicyclo[2.2.2]octane system has been used as a non-aromatic analog of the terephthalamide. Remarkably, the same hydrogen bonding patterns emerge for the diethyl amide and dimethyl amide systems. In these systems, the same three motifs are observed: chains, networks, and isolated systems.

We also have been investigating two-dimensional systems. N,N-Dialkyltrimesamides, which are derivatives of trimesic acid, inherit their trigonal symmetry from the parent acid. Three hydrogen bond-accepting groups are aligned at 120° angles from each other in the structure allowing the molecule to readily form a two-dimensional hydrogen-bond network with hydroquinone. Tetraphenylporphyrins, with amide functional groups in the para-position on the phenyl rings, create four sets of hydrogen bond acceptors that are positioned at 90° angles (Guler, 1999).
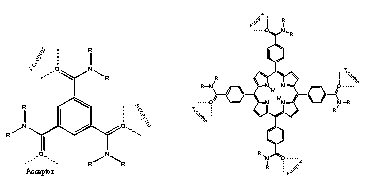
In the case of the trimesamide-hydroquinone system, again the same type of pattern has emerged with both chains and networks being formed as a result of steric accommodations of the side alkyl chains.

To extend our studies to three dimensional systems, we have looked at tetraphenyl methane and tetraphenyl adamantane systems with amide groups in the para positions (Jian, 1999; Wang, 2000). These systems have the amide hydrogen bond acceptors aligned at tetrahedral, 109.5° angles. Binary systems with hydroquinones have been investigated. So far, only alternating linear chains have been characterized, but we believe that the other motifs exist (as evidenced by phase diagrams).

Practical Applications
There have been several commercially relevant applications of this work. Diffusion control in thin films, reaction control by selective precipitation of desired products prior to secondary reactivity, controlled solubilization and release, and membrane/interface gating by selective diffusion have all been explored. Ongoing work continues to validate and explore these and other potentially useful applications.
Impact, Outreach, and Infrastructure Development
Funding from this research project provided support for the PI for summers. In addition to funding a post-doctoral associate, 9 graduate students and 18 undergraduate students have received partial financial support. This research led to more than 21 presentations by students at American Chemical Society (ACS) National Meetings, ACS Local Meetings, and other regional chemistry symposia. There have been nine completed Master’s Theses. The University of Massachusetts–Boston has not had a formal Ph.D. program in Chemistry, but beginning in September 2001, the University has approved the establishment of a Ph.D. program focusing on green chemistry. The success of the activities enabled by this prior NSF/EPA support played a large role in establishing this Ph.D. program. Five publications in journals, a book chapter, and more than 20 keynote and invited presentations at local, national, and international symposia have resulted from this funding. Also during this funding period, the PI coauthored the text Green Chemistry: Theory and Practice, Oxford University Press, 1998.
The University of Massachusetts–Boston is a 100 percent commuter school where the average age of an undergraduate student is 28. Of the 24 students funded by this project, 14 were non-Caucasian, 13 were women, and 4 were parents. As part of the PI's research program, all students have participated in K-12 and community outreach projects. These activities include visits to elementary and junior high schools, hosting visits by school groups, presentations to Girl Scout and Boy Scout troops, as well as summer programs for inner city children. As a condition of working in the PI's research group, all students are expected to be able to communicate their research objectives clearly to the non-scientific community. The PI plays an active role in the Ronald E. McNair Fellowship Program designed to serve underrepresented minority students planning to attend graduate school in the sciences. Five of the students receiving funding from this support were participants in this program.
Peripheral funding as a result of this work has been received by the University Internal Grants programs, the State of Massachusetts’ Toxics Use Reduction Program, U.S. Army Laboratories, and Los Alamos National Laboratory. This research has helped to enable the acquisition of a number on new research instruments as well as the construction of a 3,000 square foot facility: The Green Chemistry Laboratory for Research and Education in Sustainable Innovation. This combined teaching/research space houses several new research instruments whose funding was justified, at least in part, by the work performed from this prior NSF/EPA funding. In the past 4 years, a Differential Scanning Calorimeter, a Thermal Gravitometric Analysis Instrument, an Atomic Force Microscope, a scanning tunneling microscope, a nuclear magnetic resonance spectrophotometer as well as an IR, UV-vis, and HPLC have been obtained with funding from a variety of sources. The PI also has served as a Co-PI on a successful NSF grant application for a solid state NMR at the University of Massachusetts–Lowell. The PI is one of the University of Massachusetts–Boston's "Faculty for the 21st Century" members of the organization "Project Kaleidoscope," a National Program that focuses on K-16 science and Math Education Reform. As a result of the success of the research and training that has been enabled from this NSF/EPA funding, the PI has been invited to speak and hold workshops for this organization.
Based on the technical success of the research funded by this NSF/EPA funding, the PI has been asked to serve on the editorial board of two journals that focus directly on the subject of this research: Crystal Engineering and Journal of Crystal Growth and Design.
Industrial involvement has been quite satisfying. The PI's research group has formed a partnership with the Massachusetts' Executive Office of Environmental Affairs' Office of Technical Assistance. In fact, a memorandum of understanding between the University of Massachusetts (UMASS) President's Office and the Massachusetts' Secretary of the Environment has been signed to help further promote these kinds of relationships. The PI is the technology advisor to the UMASS Environmental Business Technology Center, where a strict business focus is applied to emerging technologies such as that funded by this NSF/EPA grant. Collaboration with the Polaroid Corporation, ChemMotif, Raytheon, and Gillette has helped maintain industrial practicality in this research.
References:
Foxman BM, Guarrera DJ, Taylor LD, Warner JC. Environmentally benign synthesis using crystal engineering: steric accommodation in non-covalent derivatives of hydroquinones. Crystal Engineering.1998;1:109.
Foxman BM, Guarrera PR, Tassa C, Donna J, Warner JC. Non-covalent derivatives of hydroquinone: bis-(N,N-Dialkyl)bicyclo-[2.2.2]octane-1,4-dicarboxamide complexes. Crystal Engineering 1999;2(1):55-64.
Bai, J. Steric influences in solid state complexes. M.S. Thesis, Department of Chemistry, University of Massachusetts–Boston, Boston, MA, 1998.
Tassa C. Non-covalent derivatization: evaluation of pi-interactions in self assembled systems using the amide phenol hydrogen bond. M.S. Thesis, Department of Chemistry University of Massachusetts–Boston, Boston, MA, 1998.
Guler S. Tetrakis phenylporphyrines as square planar, two dimensional components in hydroquinone complexes. M.S. Thesis, Department of Chemistry, University of Massachusetts–Boston, Boston, MA, 1999.
Jain T. Tetrahedral non-covalent derivatives, M.S. Thesis, Department of Chemistry University of Massachusetts–Boston, Boston, MA 1999.
Wang, J. Tetrahedral hydrogen bond acceptors for molecular complexation. M.S. Thesis, Department of Chemistry, University of Massachusetts–Boston, Boston, MA, 2000.
Journal Articles on this Report : 5 Displayed | Download in RIS Format
| Other project views: | All 41 publications | 10 publications in selected types | All 5 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Bui K, Warner JC. Green chemistry: deoxygenation of organic oxides via reactions with benzaldoximes. The Nucleus 1999;78:13. |
R825327 (Final) |
not available |
|
|
Cannon AS, Warner JC. Non-covalent derivatization: green chemistry applications of crystal engineering. Crystal Growth and Design 2002;2(4):255-257. |
R825327 (Final) |
not available |
|
|
Foxman BM, Guarrera DJ, Taylor LD, Warner JC. Environmentally benign synthesis using crystal engineering: steric accommodation in non-covalent derivatives of hydroquinones. 1998; 1(1): 109-118. |
R825327 (Final) |
not available |
|
|
Foxman BM, Guarrera PR, Tassa C, Donna J, Warner JC. Noncovalent derivatives of hydroquinone: bis(N,N-dialkyl)bicyclo[2.2.2]octane-1,4-dicarboxamide complexes. Crystal Engineering 1999;2(1):55-64 |
R825327 (Final) |
not available |
|
|
Warner JC. Green Chemistry: The Frontier of Pollution Prevention. Massachusetts Environmental Ventures 1999;3(1):1 |
R825327 (Final) |
not available |
organics, green chemistry, waste reduction, innovative technology, environmental chemistry, alternative chemical synthesis, chemical processing, cleaner production, environmentally friendly chemical synthesis, innovative technology, molecular assemblies, non-covalent derivatization, pollution prevention, process modification, source reduction, supermolecular structures. , Sustainable Industry/Business, Scientific Discipline, Chemical Engineering, cleaner production/pollution prevention, Environmental Chemistry, process modification, chemical processing, cleaner production, environmentally-friendly chemical synthesis, in-process changes, waste reduction, molecular assemblies, non-covalent derivatization, green chemistry, sustainable development, alternative chemical synthesis, source reduction, innovative technology, pollution prevention
Relevant Websites:
http://www.greenchemistry.umb.edu ![]()
Progress and Final Reports:
Original Abstract
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20090825071006im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)