Testimony
Statement by
Julie L. Gerberding, M.D., M.P.H.,
Director
on
Extensively Drug Resistant TB: CDC’s Public Health Response
before
Committee on Foreign Affairs
Subcommittee on Africa and Global Health
U.S. House of Representatives
Wednesday, March 21, 2007
Good afternoon, I am Dr. Julie Louise Gerberding, Director of the Centers for Disease Control and Prevention within the Department of Health and Human Services (HHS). It is my pleasure to be here to discuss with you CDC's role in the response to extensively drug resistant TB, globally and in the United States.
Definition
Tuberculosis (TB) is an airborne infectious disease that is spread from person to person, usually through coughing. In the late 19th and early 20th centuries, until the introduction of streptomycin in the forties, TB was one of the leading causes of death in the United States. Currently, the World Health Organization (WHO) reports that one in three people in the world is infected with dormant TB germs (i.e. TB bacteria). Only when the bacteria become active do people become ill with TB. Bacteria become active as a result of anything that can reduce the person's immunity, such as HIV, advancing age, or some medical conditions. Currently TB that is not resistant to drugs can be treated with six to nine months of "first-line drugs" (the most effective), including isoniazid and rifampin; this treatment cures over 95 percent of patients. However, since people in many resource-poor countries lack access to appropriate treatment, nearly nine million people in the world develop TB disease each year and about 1.6 million die.
TB that is resistant to at least isoniazid and rifampin is called multidrug-resistant (MDR) TB. MDR TB requires treatment for 18-24 months with "second-line drugs" that are much less effective, poorly tolerated by the patient, and far more costly. There are currently only six second-line drugs, of which two-fluoroquinolones and injectable macrolides-are the most important. The cure rate is 70-80 percent under optimal conditions, but is usually closer to 50 percent. Many countries with a high TB burden find it impossible to treat MDR TB patients because of the cost of drugs, and the more sophisticated laboratory services and more intensive programmatic support required for administering them. Extensively drug-resistant TB (XDR TB) is a subset of MDR TB caused by strains of bacteria that are resistant to the most effective first- and second-line drugs.
Causes
Drug resistance develops when patients receive incomplete or inadequate treatment. Persons with these resistant strains in their lungs can then pass these resistant bacteria to other susceptible individuals through coughing. We have also learned that weaknesses in a TB program create opportunities for drug resistance to develop: either through the interruption of drug supply, the inappropriate prescription treatment regimens administered by medical providers, the failure to support patients on therapy, the non-adherence to treatment by patients, and the lack of implementation of infection-control precautions.
Scope of the problem
In response to anecdotal reports from physicians who were finding cases of TB that were unresponsive to the first-line and second-line TB drugs, in 2005 the CDC and the WHO jointly conducted a survey, with support from the U.S. Agency for International Development, that examined about 18,000 patient isolates tested during 2000 to 2004 by Supranational Reference Laboratories. Researchers examined the drug-resistant isolates, and found that 10 percent of the MDR TB isolates actually met the definition for XDR TB. XDR TB was identified in 17 countries from all regions of the world, most frequently in the former Soviet Union and Asia. Data from sub-Saharan Africa were very limited because of poor laboratory capacity within the region as a whole. More representative data showed that in the United States, 2.9 percent of MDR TB cases were XDR TB; in the Republic of Korea, 15 percent of the MDR TB cases were XDR TB; and in Latvia ,19 percent of MDR TB cases were XDR TB. This report, published in CDC's Morbidity and Mortality Weekly Report in March 2006, was the first widely circulated publication to use the term "extensively drug resistant TB" was widely published. FIGURE 1 shows the countries found to have XDR TB in the joint HHS/WHO survey.
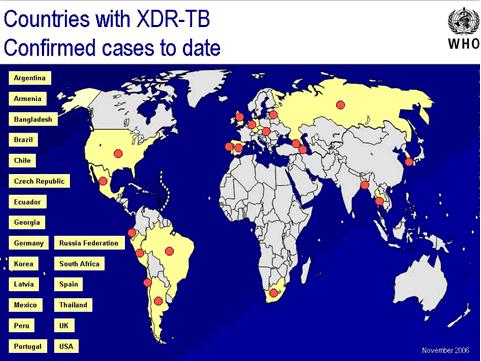
Note: Cases from 2001 through 2004; Red dots represent countries where XDR TB has been identified.
Because many countries do not routinely test all isolates for resistance to second line drugs, the precise global incidence of XDR TB remains uncertain. However, because of the ease with which drug resistance can occur (because of the use of second-line drugs in suboptimal conditions, funding shortages, changes in program focus away from TB case management, interruptions in drug availability, high HIV prevalence), XDR TB could be much more widespread than this survey shows.
Morbidity and Mortality from XDR TB
Reported mortality rates among persons with XDR TB are extremely high. Among non-HIV infected persons, reports indicate that less than 30 percent of patients can be cured, and more than half of those with XDR die within five years of diagnosis. Among HIV-infected persons, illness is more severe, and mortality rates are higher and death occurs within a shorter time. Not long after the publication of the CDC-WHO survey results, we learned of a deadly outbreak of XDR TB among HIV-infected TB patients in KwaZulu-Natal Province in the Republic of South Africa.
In this outbreak, of the 544 patients affected with TB, 221 (41%) had MDR TB, and 53 of those met the definition of XDR TB. All but one of the XDR TB infected persons died, (a mortality rate of 98 percent), with a median survival period of only 16 days from the time healthcare workers collected their sputum for analysis. Mortality among those with MDR TB received less attention, but was also high--in excess of 70 percent. Among those patients with XDR TB, 55 percent had no prior history of TB treatment, which indicated they had contracted their TB directly from other infected individuals. Forty-four of the XDR TB patients were also underwent testing for HIV, and 100 percent were found positive. Of these, 15 were receiving antiretroviral therapy, which is an important caution as we succeed in providing anti-retroviral therapy to more and more HIV-infected persons. Given that TB is still a threat to HIV-infected persons, the President's Emergency Plan, the Global Fund to Fight HIV/AIDS, Tubercluosis and Malaria, national governments and other partners must ensure programs to prevent and control TB work closely together to protect vulnerable populations from acquiring this virtually untreatable form of TB.
What is the threat in the United States?
The TB resurgence that occurred from 1985-1992 in our country provides an excellent example of how outbreaks of drug-resistant TB can develop. From 1953 (the establishment of current US surveillance practices) through the mid 1980's, TB cases in the United States declined steadily, from approximately 83,000 to 22,000 new cases per year. But in 1985, CDC began documenting increases in TB incidence. Factors associated with this increase include the dismantling of TB programs, which occurred when health departments stopped receiving TB categorical funds, and shifted resources to other public-health activities. Other factors included the burgeoning HIV epidemic, increased immigration from countries with high TB incidence rates, lack of infection-control precautions in healthcare settings, and the widespread occurrence of MDR TB at a time when the laboratory capacity to readily identify these strains was inadequate. The Congress then appropriated an increase in funds, and the situation was remedied once programs were again able to prescribe appropriate drug regimens for patients, have adequate laboratory capacity to diagnose and manage patients, provide appropriate programmatic support for patients, assure adherence with prescribed regimens, and conduct effective contact investigations.
These intensive control efforts also resulted in a decrease in MDR TB cases in the United States, which fell from approximately 400 per year to about 100 per year. However, the epidemiology of these cases also changed; in 1993, 26 percent of MDR TB cases in the United States occurred in foreign-born persons; whereas in 2005, 80 percent of MDR TB cases occurred in foreign-born persons. Between 1993 and 2006, 49 cases of XDR TB were reported in the United States to CDC. As with MDR TB, the epidemiology for XDR has changed remarkably over that time period. In the years 1993-1999, 19 (59 percent) of XDR TB cases occurred in U.S.-born persons and 44 percent occurred in persons with HIV infection. In the years 2000-2005, 80 percent of XDR TB cases occurred in foreign-born persons and only 13 percent occurred in HIV-infected persons. While the total number of MDR and XDR TB cases is relatively small, their impact on U.S. TB control programs can be significant in terms of human capital and financial resources. One patient with MDR or XDR TB requires a minimum of 18-24 months of treatment. Recently collected data show that in-patient costs alone are $500,000 per case.1 The treatment of some individual cases has cost as much as $1 million. The cost of a potential resurgence, however, is far higher. In New York City alone, the estimated cost to control the MDR TB epidemic of the late 1980's exceeded one billion dollars (in 1991-adjusted dollars).2
One of the first-line of defense to prevent importation of TB into the United States is the overseas medical screening of immigrants. With the cooperation of the U.S. Department of State and international partners, HHS/CDC is in the process of implementing improved screening procedures (including both cultures and drug-susceptibility testing) that, according to preliminary studies, are three times as sensitive at detecting TB. Individuals identified with active TB are now must complete their treatment before they leave to start their lives in the United States.
MDR and XDR in high HIV-prevalence areas
In areas such as sub-Saharan Africa, TB rates have substantially increased over the past decade, which parallels the rising number of HIV/AIDS immuno-compromised patients, and makes it more difficult to diagnose and treat TB. More than 50 percent of the persons with TB in sub-Saharan Africa are HIV-infected. In countries with a high HIV burden, weak and underfunded TB Control Programs become strained by the influx of new TB patients. In most of these countries, the government does not regulate second-line drugs and they are not widely available. In Botswana, for example, TB incidence was declining until about 1987, when it began to rise sharply as HIV prevalence increased, (as measured by a study of women attending antenatal clinics), tripling by 2002. A significant increase in the prevalence of overall drug resistance among the TB cases followed this jump in the burden of TB patients. The WHO and its partners anticipate that drug resistance in this setting will increase, because of the weakness of the national TB programs in many countries.
MDR and XDR TB in countries with low HIV prevalence
XDR TB is also a potentially dangerous problem for countries with low HIV prevalence if they do not have adequate national TB programs. The necessary conditions for programs to "grow" resistant TB occurs where physicians routinely prescribe drug regimens were routinely prescribed without the benefit of drug susceptibility testing. Available data indicate the highest MDR TB and XDR TB prevalence occur in Eastern Europe and Asia in low-HIV-prevalence populations. Persons in these countries who are treated effectively are cured of non-resistant TB, but if conditions exist in which MDR TB is created, then the necessary widespread use of second-line drugs can rapidly foster development of XDR TB as well. For example, an anesthesiologist in Russia developed MDR TB after caring for a patient who had highly drug resistant TB. She died soon after diagnosis, despite treatment with second-line TB drugs. As HIV spreads among these patients and other control conditions are not adequate, a country may face an outbreak of untreatable TB.
Response to XDR TB Globally
CDC works closely with other agencies to prevent TB globally, including the National Institutes of Health (NIH), other federal entities like the U.S. Agency for International Development (USAID), the WHO and non-governmental agencies through a variety of programs, including the Emergency Plan and the Global Fund for AIDS, TB and Malaria. In September 2006, HHS/CDC, WHO, and other partners from the Stop TB partnership developed an action plan to address XDR TB. This includes taking the first, all-important step of addressing TB program deficiencies as quickly as possible to "turn off the faucet" of drug resistance. The action plan recommended the following:
- Conduct rapid surveys of XDR TB to determine the burden of disease;
- Enhance laboratory capacity to support surveillance and diagnosis, with emphasis on drug-susceptibility testing;
- Improve the technical capacity of practitioners to respond to XDR TB outbreaks and manage patients;
- Implement infection-control precautions;
- Increase research support to develop new anti-TB drugs;
- Increase research support to create rapid diagnostics for TB and for MDR and XDR TB; and
- Promote universal access to antiretrovirals under joint TB/HIV activities
As an initial step, the U.S. Federal TB Task Force has been discussing a domestic and international response plan for U.S. Government agencies on XDR TB. The U.S. Government also participated in the WHO Global XDR TB Task Force that has issued a global plan to respond to XDR TB. The White House will convene an interagency meeting in the next few weeks to ensure U.S. Government activities are integrated in a unified strategic approach.
HHS/CDC also supports WHO and the Stop TB Partnership on a number of important activities, including technical assistance to the Global Drug Facility, which works to supply quality medications for TB programs, and the Green Light Committee, which supports efforts to develop high-quality appropriate, managed access to drugs for MDR TB.
In addition, HHS/CDC's TB Trials Consortium has the leading role in clinical tuberculosis research. Results from these trials have formed the basis for the Treatment Guidelines developed by HHS/CDC and the American Thoracic Society's, and in updating regimens for both HIV and non-HIV infected patients. This research will be increasingly important for the development of new drugs and regimens for drug-resistant TB will be required.
CDC technical experts are also working directly with host country governments and partners to urgently implement improved infection control, rapid case detection, effective treatment, surveillance for drug resistance, and expanded program capacity, on an urgent basis. For example, CDC employees recently assisted colleagues in South Africa by providing technical support in training activities to implement infection-control measures. CDC has also assembled teams of experts, including epidemiologists, microbiologists, and infection control specialists who are prepared for rapid deployment in response to XDR TB outbreaks throughout the world.
Response to XDR in People Living with HIV/AIDS
With the support of the Office of the Global AIDS Coordinator (OGAC) and PEPFAR funding, CDC has been providing technical assistance to host governments in PEPFAR-supported countries. This funding has been used to strengthen collaboration between National TB and AIDS Control Programs and to work with National Public Health Laboratories to strengthen TB diagnostic services. This technical assistance supports a variety of activities, including (1) decreasing the pool of severely immunocompromised patients through ARV treatment, (2) reducing TB morbidity and mortality through early identification of TB suspects and patients in HIV prevention and care settings, (3) integrating TB and HIV services to assure uninterrupted treatment of HIV-infected TB patients, and (4) providing isoniazid preventive therapy as part of a package of care for HIV-infected patients. In addition, CDC has helped to strengthen TB lab capacity, especially at points of service to promote rapid diagnosis of TB; conduct TB drug resistance surveillance; and strengthen TB infection control practices in HIV care settings. In FY 2007, a portion of PEPFAR funds will be used to address prevention and control of XDR TB in HIV-infected persons.
Gaps
HHS/CDC, WHO, and USAID have taken critical steps toward characterizing and controlling the threat of XDR TB. For example, considerable improvement in TB infection-control practices in healthcare settings can be achieved through relatively simple and inexpensive practices (for example, having waiting rooms outside in covered but open areas, installing fans, separating coughing patients, etc.) can achieve considerable improvements in TB infection-control practices in healthcare settings. To provide guidance on TB infection control, CDC, in collaboration with the WHO, OGAC, and the International Union Against TB and Lung Disease has recently published a guidance document titled "TB Infection Control in the Era of Expanding HIV Care and Treatment," now available on the CDC website.
A larger commitment is required in other areas, especially diagnostic services, treatment, and program management. Research on new tools for prevention, treatment, and diagnosis are needed both domestically and internationally to modernize and accelerate TB elimination. Importantly, the international community lacks new, effective drug regimens to replace drugs that have become ineffective against TB, or that interact unfavorably with anti-retrovirals and other HIV medications. According to the Advisory Council for the Elimination of Tuberculosis, TB drug development is at an unprecedented point. For the first time in 50 years at least four new anti-TB compounds entered human clinical trials, and several others are ready for advanced pre-clinical testing. These new compounds represent new drug classes that are not cross-resistant with existing agents, and can offer promise for resistant cases.
New diagnostic tests for TB are also needed. Currently diagnosis of TB disease relies on the sputum smear examination, which has been in use for 125 years and is poorly sensitive and highly inefficient. New blood tests have entered the market recently, and appear to offer slightly improved performance, although they are more costly and have not undergone evaluation in the programmatic setting. Field evaluation of optimal, efficient diagnostic tests, as well as rapid tests for the detection of TB drug resistance, is critical. Globally, there is limited laboratory capacity for TB and drug-resistance testing.
The global XDR TB response has highlighted the need for laboratories to make services for TB, MDR TB and XDR TB more rapid, sensitive, and reliable. TB patients in developing countries lack access to reliable, quality-assured, and prompt TB laboratory services. As a result, clinicians are unable to make correct patient management decisions. Many laboratory techniques used to confirm a diagnosis of TB and to identify drug resistance were developed in the 1950's, 60's, and 70's. To combat resistance to anti-TB drugs, clinicians must have the most current methods, applied to their fullest capacity. Increasing the availability of genotyping also would allow programs to identify links between patients.
In addition, given the increasing proportion of the burden of TB in the United States among foreign-born persons, there is a strong need to improve the quality of overseas medical screening of U.S. bound immigrants, including the ability to detect and treat XDR TB in this population.
Equally important will be the strengthening of program infrastructures, both domestically and abroad, through training and sustained support. Strong program infrastructure will prevent new agents from becoming drug-resistant.
Thank you for the opportunity to present CDC's findings and activities on XDR TB to date. I would be happy to answer any questions.
1 Inpatient care has been estimated for California XDR TB patients from 1993-2006 at an average of approximately $600,000 per patient. These estimates do not include outpatient costs or productivity losses, which are likely to be substantial for those treated for may years, or for the 25 percent of whom died from XDR TB. Jenny Flood, MD, TB Controller, State of California, personal communication.
2 (Tuberculosis in New York City - Turning the Tide Thomas R. Frieden, M.D., M.P.H., Paula I. Fujiwara, M.D., M.P.H., Rita M. Washko, M.D., and Margaret A. Hamburg, M.D. New England Journal of Medicine, July 27, 1995).
Last revised: March 26,2009