Welcome to the winter 2008 edition of Inside NIMH. Over the past few months, the National Institute of Mental Health (NIMH) has been involved in several noteworthy activities, which I am pleased to share with you in this newsletter.
Sincerely,
Thomas R. Insel, M.D.
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your e-mail address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page.

March 2008 Contents
I. Message from the NIMH Director
In this edition of the newsletter, I would like to update you about the NIMH budget and funding policy for Fiscal Year (FY) 2008 and the strategies we are implementing to help maintain funding levels. I will also describe a recent change to the NIH Public Access Policy and discuss enhancing the peer review system at NIH.
On December 26, 2007, the FY 2008 Omnibus appropriations bill was signed. The NIH program level is $29.5 billion, about $648 million more than the FY 2007 Revised Joint Resolution. The FY 2008 appropriation for NIMH is $1,405 million—an increase of $982 thousand or +0.1 percent over the FY 2007 Joint Resolution.
This level of funding marks the fifth consecutive year that the NIH and NIMH budget grew at a rate less than biomedical research inflation, estimated to be 3.5 percent per year. During this same time, the number of Research Project Grant (RPG) applications increased by nearly 27 percent (2,210 in FY 2003 to 2,803 in FY 2007). To help us weather the difficult budget environment, NIMH implemented key management strategies: controlling the average size of RPGs, emphasizing Institute priorities in funding decisions, and focusing on opportunities for innovation and new Principal Investigators (PIs). Dividends from implementing these strategies are paying off. In FY 2007, NIMH was able to fund 620 Competing RPGs and increase the success rate for RPGs to 22.1 percent (up from 19.7 percent in FY 2006).
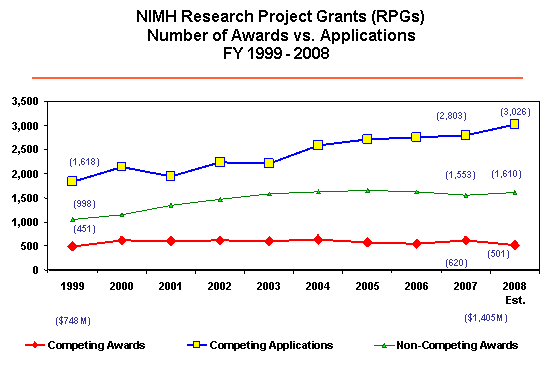
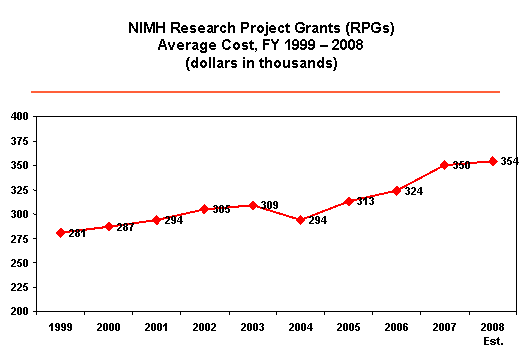
What does this mean for NIMH in FY 2008? NIMH will continue to make strategic adjustments and careful funding decisions. NIMH’s priorities include our continued commitment to funding new PIs and innovative science, and our priorities for new competing awards remain similar to FY 2007. We also continue to modify and more actively contain costs in non-competing awards. Even with these strategies in place, it will be difficult to maintain the funding levels from FY 2007, especially as the costs of research continue to rise and as more researchers apply for NIMH grants. For FY 2008, NIMH expects to see an 8 percent increase in the number of RPG applications from FY 2007, and a rise in the average cost of RPGs by approximately $4,000 over FY 2007 numbers. As a result, NIMH anticipates that the number of new awards for Competing RPGs will decrease from 620 in FY 2007 to approximately 501 in FY 2008. To continue transparency in communicating our funding strategy, NIMH posted its guidelines for paying research grant awards issued during the period October 1, 2007, through September 30, 2008. I encourage you to gain more details by reviewing the FY 2008 Funding Strategy for Research Grants.
I would also like to inform you about an important change to the NIH Policy on Enhancing Public Access to Archived Publications Resulting from NIH-Funded Research due to recent Congressional legislation. As of January 2008, the NIH Public Access Policy now requires scientists to submit journal articles that arise from NIH funds to PubMed Central (PMC), the NIH digital archive of full-text, peer-reviewed journal articles. The Policy requires that these articles be accessible to the public on PMC to help advance science and improve human health.
The NIH Public Access Policy applies to all peer-reviewed articles that arise, in whole or in part, from direct costs funded by NIH, or from NIH staff, that are accepted for publication on or after April 7, 2008. In addition, beginning May 25, 2008, anyone submitting an application, proposal or progress report to the NIH must include the PMC or NIH Manuscript Submission reference number when citing applicable articles that arise from their NIH funded research. This policy includes applications submitted to the NIH for the May 25, 2008, due date and subsequent due dates. I encourage you to view the revised policy for additional details and to consider offering comments to NIH on implementation of the revised policy. See the Notice of Public Meeting: Seeking Comments on Implementation of the NIH Public Access Policy.
Finally, I would also like to encourage you to review and comment on the NIH 2007-2008 Peer Review Self-Study Final Draft Report (PDF File), which identifies the most significant challenges facing the NIH peer review system and proposes recommended actions. If you wish to comment on the Final Draft, please send your comments no later than Monday, March 17, 2008, to PeerReviewRFI@mail.nih.gov or via U.S. Mail (address shown at the NIH Web site on Enhancing Peer Review.)
II. New Announcements about Funding Opportunities
Each week, NIH electronically distributes the NIH GUIDE, a listing of all NIH Funding Opportunity Announcements (FOAs), which include requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below are samples of FOAs in which NIMH participates. The Research Funding page on the NIMH Web site has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to regular weekly e-mails of the NIH GUIDE.
NIMH-Administered Requests for Applications
Limited Competition for Data Deposition and Analyses of Genome Wide Association Studies of Mental Disorders
This initiative requests applications for conducting secondary statistical analysis on existing whole genome association data sets for genetic studies of five major mental disorders including attention-deficit hyperactivity disorder (ADHD), Bipolar Disorder, Autism, Major Depression Disorder (MDD) and Schizophrenia. The applicants are required to be participants in the recently formed Psychiatric GWAS Consortium (PGC) and to propose the performance of innovative analyses on combined data sets across different genotyping platforms and across phenotypes using existing or newly developed statistical methods.
Release Date: February 28, 2008; Expiration Date: May 1, 2008
- NIH GUIDE version of the R01 announcement (RFA-MH-08-120)
- NIH GUIDE version of the Collaborative R01 announcement (RFA-MH-08-121)
Genomic Parsing of Bipolar Disorder and Schizophrenia: Studies of Large Cohorts in the U.S. and Across the Globe
This initiative seeks applications that propose to enrich pre-existing resources for schizophrenia and bipolar disorder in the NIMH Human Genetics Initiative and to apply genomic and related research tools to further our understanding of the molecular etiology of these disorders. This FOA will use the NIH Research Project Grant (R01) award mechanism and runs in parallel with an FOA of identical scientific scope, RFA-MH-08-131, that solicits applications under the Collaborative Research Project Grant (Collaborative R01) funding mechanism.
Release Date: March 4, 2008; Expiration Date: May 7, 2008
- NIH GUIDE version of the R01 announcement (RFA-MH-08-130)
- NIH GUIDE version of the Collaborative R01 announcement (RFA-MH-08-131)
Basic and Translational Research Opportunities in the Social Neuroscience of Mental Health
NIMH and the National Institute on Aging invite applications that examine the neurobiological bases of social behavior — including its genetic, developmental, cognitive and affective components — at either the basic or translational levels of analysis. It is anticipated that findings derived from these approaches will ultimately aid in understanding of the causes or disease courses of mental disorders, or will add to the knowledge base necessary for developing appropriate biomarkers or identifying key endophenotypes that will further advance the understanding of the causes and treatments of mental disorders across the developmental lifespan.
Release Date: August 8, 2007; Expiration Date: October 21, 2008
- NIH GUIDE version of the R01 announcement (RFA-MH-08-070)
Innovative Approaches to Personalizing the Treatment of Depression
NIMH solicits grant applications for research to develop innovative approaches to personalizing treatment for patients suffering from depression. While effective treatments of depression are available, there is considerable individual variation in treatment response. Progress has been made in identifying potential predictors and moderators of treatment effects, but no specific model for individualizing care has been developed or tested for efficacy. The purpose of this FOA is to advance research on individualizing the treatment of depression by developing models and testing new approaches that, by accounting for patient characteristics, aim to be more specific and thus potentially leading to more effective and efficient treatment interventions. Consistent with the current state-of-the-science in this relatively new area of research, this FOA is meant to support relatively small projects or preliminary investigations, rather than large studies that definitively test intervention effectiveness. This FOA is therefore an initial step toward advancing research on personalization of care in mental health.
Release Date: March 7, 2008; Expiration Date: June 21, 2008
- NIH GUIDE version of the R01 announcement (RFA-MH-09-010)
- NIH GUIDE version of the R34 announcement (RFA-MH-09-011)
Novel Interventions for Neurodevelopmental Disorders
This FOA solicits R34 grant applications that propose to develop novel interventions that will improve functioning in domains commonly affected by neurodevelopmental disorders. It provides resources for evaluating the feasibility, tolerability, acceptability and safety of novel approaches, and for obtaining the preliminary data needed to support the development of a larger-scale efficacy trial. This FOA is intended to encourage a broad scope of new treatment approaches with potential for widespread, cost-effective application across a variety of neurodevelopmental disorders based on the targeted domain(s) of functioning. The treatment approaches may include psychopharmacologic, other physiological, cognitive, psychosocial, psychoeducational, and behavioral approaches or novel combinations of any of these approaches. Also of interest are particularly innovative and theoretically-based intervention approaches currently in clinical use that have not been adequately evaluated for safety and efficacy.
Release Date: March 7, 2008; Expiration Date: June 18, 2008
- NIH GUIDE version of the R34 announcement (RFA-MH-09-020)
- NIH GUIDE version of the R21/R33 announcement (RFA-MH-09-021)
Prevention of Trauma Related Adjustment and Mental Disorders in High-Risk Occupations
NIMH invites applications that will contribute directly to the goal of establishing empirically-demonstrated methods of preventing the development of trauma-related disorders among high trauma exposure occupational groups, for example, civilian employees and military personnel who regularly encounter traumatic situations. From a scientific perspective, occupations that involve exposure to trauma at higher than average frequency present unique opportunities for testing the effectiveness of preventive interventions designed to minimize posttraumatic adjustment disorders. From a public health and national security perspective, attending to the mental and behavioral health of individuals and groups who respond to emergencies, provide disaster relief, defend national interests, participate in peacekeeping missions, and maintain a civil society can be viewed as strengthening our national infrastructure. This RFA is being issued under the R01 and R34 mechanisms.
Release Date: April 12, 2007; Expiration Date: November 22, 2008
- NIH GUIDE version of the R01 announcement (RFA-MH-08-010)
- NIH GUIDE version of the R34 announcement (RFA-MH-08-011)
NIMH-Collaborative Requests for Applications
Research on HIV/AIDS and Drug Use in the Multi-center AIDS Cohort Study (MACS)
This program fosters the use of small research grants (R03s) to build on an existing resource, the MACS. The National Institute on Drug Abuse will support research on clinical epidemiology related to drug use and HIV/AIDS, socio-behavioral, neuroAIDS, and medical consequences that use pilot and feasibility studies; secondary analysis of existing data; self-contained research projects; collaborative studies linked to larger research projects; studies of statistical methodologies and modeling; and studies of new assessments and research techniques. NIMH will support similar types of studies that focus on HIV, the central nervous system, pharmacotherapeutics, and biomarker development strategies.
Release Date: November 16, 2007; Expiration Date: March 15, 2008
- NIH GUIDE version of the R03 announcement (RFA-DA-08-008)
Nutrition and Prevention, Care, and Treatment of HIV/AIDS
This FOA is intended to stimulate and strengthen a multidisciplinary approach to a complex, under-researched area and to form a basis for future research and clinical care. Specifically, the National Institute of Child Health and Human Development and NIMH invite new and experienced basic scientists, epidemiologists, and clinical investigators to submit research grant applications to further understanding of the relationship between nutrition and HIV infection. Such research will support the integration of nutrition into all aspects of care and treatment of people infected and affected by HIV/AIDS. Consequently, research grant applications are encouraged that address preclinical or clinical, biomedical and/or behavioral research.
Release Date: January 10, 2008; Expiration Date: April 11, 2008
- NIH GUIDE version of the R01 announcement (RFA-HD-07-022)
Integration of Food and Nutrition into Prevention, Care, and Treatment of HIV Infection and AIDS
This FOA solicits Small Grant (R03) applications from domestic or foreign institutions that propose to expand our appreciation of the scope of nutritional problems in the context of HIV across various regions hard hit by the epidemic and to evaluate the impact of new guidelines (e.g., WHO Guidelines for nutritional care of people living with HIV and AIDS; PEPFAR Policy Guidance on the Use of Emergency Plan Funds to Address Food and Nutrition Needs) regarding use of food and nutrition support in programs designed to address HIV prevention, progression, care, and treatment. This FOA is intended to stimulate and strengthen a multidisciplinary approach to a complex, under-researched area and to form a basis for future research and clinical care.
Release Date: January 10, 2008; Expiration Date: April 11, 2008
- NIH GUIDE version of this R03 announcement (RFA-HD-07-023)
Methods for Prevention Packages
This FOA is intended to encourage collaborations between behavioral and biomedical clinical scientists, epidemiologists, mathematical modelers, and clinical trial design specialists to develop new research strategies and methodologies that will facilitate the design and testing of combination HIV prevention interventions (“prevention packages”).
Release Date: February 21, 2008; Expiration Date: July 16, 2008
- NIH GUIDE version of the R01 announcement (RFA-AI-08-019)
Integrated Preclinical/Clinical Program for HIV Topical Microbicides (IPCP-HTM)
The National Institute of Allergy and Infectious Diseases and NIMH invite applications from single institutions and consortia of institutions to participate in this FOA for the advancement of novel single and combination safe, effective and acceptable microbicides and microbicide strategies to prevent sexual transmission of HIV. The types of microbicide research that will be supported include basic microbicide science, focused preclinical development and exploratory small scale clinical trials (pre-Phase I clinical trials). The IPCP-HTM is specifically designed to serve as a platform for microbicide development through support for integrated and iterative research projects and activities including but not limited to: microbicide-relevant basic science; drug discovery-driven development of microbicides; preclinical virologic and toxicologic assessment of lead candidates; development and validation of Good Laboratory Practice (GLP)-compliant analytical assays; and Good Manufacturing Practice (GMP)-manufacturing activities in support of pre-Phase I clinical trials. Applications may include any combination of these activities.
Release Date: March 4, 2008; Expiration Date: July 18, 2008
- NIH GUIDE version of the U19 announcement (RFA-AI-08-006)
NIMH Program Announcements
Since the beginning of November 2007, NIMH has published several program announcements highlighting areas of research interest, which span topics in genetics, basic neuroscience, behavioral science, translational research, interventions, and mental health services research. The NIMH Web site has a full listing of these program announcements.
NIH Roadmap Initiatives
The NIH Roadmap is a set of transdisciplinary initiatives that seeks to transform all of biomedical research and accelerate its discoveries. All NIH Institutes, including NIMH, participate in the Roadmap, and funding opportunities are open to all investigators. Currently, workgroups co-chaired by the Directors of NIH Institutes and Centers (ICs) and populated by nominees from interested Institutes are developing initiatives for “Roadmap 1.5.” A full summary of Roadmap activities can be found at the NIH Roadmap site.
Epigenetics
The NIH Roadmap Epigenomics Program focuses on researching the origins of health and susceptibility to disease that are, in part, the result of epigenetic regulation of the genetic blueprint. This program will transform biomedical research by developing comprehensive reference epigenome maps and developing new technologies for comprehensive epigenomic analyses. A Technical Assistance Workshop was held in December to address questions and concerns regarding funding opportunities under this program. For information on epigenetics initiatives, see the NIH Roadmap Epigenomics Funding Opportunities Web page.
Human Microbiome Project
The NIH Roadmap initiated the Human Microbiome Project (HMP) to generate resources enabling comprehensive characterization of the human microbiome and analysis of its role in human health and disease. The NIH HMP continues the practice established by the Human Genome Project of international collaboration to create a comprehensive and publicly available data set. A recently issued FOA includes:
Human Microbiome Demonstration Projects
This FOA invites applications for projects that will examine, through molecular approaches, the relationship between changes in the human microbiome and human health and disease. This program is a component of the NIH Roadmap 1.5 Human Microbiome Project. The program will be funded in two phases. The initial one-year pilot phase (UH2) will allow investigators to procure samples and generate data that will be used to support the scale-up phase (UH3), which will be awarded after administrative review to those projects that have the most promise to demonstrate whether or not the human microbiome plays an important role in human health and disease. The total amount of funds available for these awards is approximately $4 million total costs for the UH2 in FY 2009 and $24.3 million for the UH3 in FY 2010-12. Up to 10 UH2 and 5 UH3 awards are anticipated from this solicitation. Awards issued under this FOA are contingent upon the availability of funds and the submission of a sufficient number of meritorious applications. See the NIH GUIDE notice for eligible institutions/organizations and eligible Project Directors/Principal Investigators.
Release Date: December 4, 2007; Expiration Date: May 23, 2008
- NIH GUIDE version of the UH2/UH3 announcement (RFA-RM-08-012)
More information on these initiatives can be found at NIH Roadmap Human Microbiome Project Funding Opportunities Web page.
NIH Neuroscience Blueprint Initiatives
The Neuroscience Blueprint is a framework to enhance cooperative activities among 15 NIH Institutes and Centers that support research on the nervous system. The Blueprint aims to develop research tools, resources, and training and to make them available to the neuroscience community. The Blueprint will focus on neural development in 2008 and neural plasticity in 2009, with plans to continue the Blueprint initiative beyond FY 2009.
III. Future Research Directions
National Advisory Mental Health Council (NAMHC) Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects.
To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- Novel Interventions for Neurodevelopmental Disorders
- Networks for Developing PTSD Risk Assessment Tools
- Novel NeuroAIDS Therapies: Integrative Preclinical/Clinical Program
- Recovery After an Initial Schizophrenic Episode
- Limited Competition for Data Deposition and Analysis of Genomic Wide Association Studies of Mental Disorders
- Genomic Parsing of Bipolar Disorder and Schizophrenia: Studies of Large Cohorts in the US and Across the Globe
Related Information
- Past NAMHC Meetings (contains links to agendas, minutes, and Director’s Reports)
- A listing of recent NAMHC-approved concepts
Upcoming NIMH Co-Sponsored Meetings
NIMH is co-sponsoring NCDEU 2008: New Research Approaches for Mental Health Interventions, May 27-30, 2008 in Phoenix, Arizona, where presenters will focus on the most important developments in psychopharmacologic clinical research. See the NCDEU Web page for information about submissions and the new features for the 2008 meeting.
Summaries of NIMH-Sponsored Scientific Meetings
Research workshops and scientific meetings are some of the best forums in which to identify research gaps and to stimulate new areas of mental health research. Below are brief descriptions of meetings that NIMH has sponsored over the past several months. You should send questions about a specific meeting to the program contact listed in the description.
- Child and Adolescent Effectiveness Research in Clinical Practice and Community Settings: Needs, Challenges, and Opportunities
- Design and Evaluation of Clinical Trials for PTSD: A VA, NIMH, DOD Working Group
- Strategies for Developing Novel Interventions for Neurodevelopmental Disorders
- NIH Conference on Building the Science of Dissemination and Implementation in the Service of Public Health
- Translational Approaches to Studying Repetitive Behavior and Resistance to Change in Autism
IV. Update on Electronic Submission of Grant Applications
NIH continues to have success with the electronic submission process for grant mechanisms that have converted so far, including R01s, R03s, R21s and R34s. Transitions are still on hold for Career Development (K), Fellowship (F), Training & Development (T&D) and complex mechanisms (such as “P” Center mechanisms). The standing submission dates for February-March 2008 will use PureEdge as previously indicated. NIH plans to test the revised, Adobe-based forms with a few FOAs this spring and is scheduled to complete testing in early 2008. If all goes well with the initial NIH FOAs, a full transition to Adobe will quickly follow. Grants.gov is encouraging agencies to complete their transitions to the new Adobe forms by early summer.
The Grants.gov site has links to the viewer download and system requirements on their Apply for Grants Web page. Also, testing is underway to ensure that the new Grants.gov 2007 system displays application images properly. Transitions of some remaining grant mechanisms are expected to resume in mid 2008. For more information, see the Electronic Submission Web site. You can also subscribe to the NIH Electronic Receipt Listserv to receive periodic updates on the electronic grant application program.
V. Recent NIMH News Releases
- One Gene Overrides Another to Prevent Brain Changes that Foster Depression (March 12, 2008)
- Bipolar Youths’ Misreading of Faces May be Risk Marker for Illness (March 4, 2008)
- Teens with Treatment-resistant Depression More Likely to Get Better with Switch to Combination Therapy (February 26, 2008)
- Genetic Tags Reveal Secrets of Memories’ Staying Power in Mice (February 21, 2008)
- Gene Variants Protect Against Adult Depression Triggered by Childhood Stress (February 4, 2008)
- Autism Risk Higher in People with Gene Variant (January 10, 2008)
- Scientists Can Predict Psychotic Illness in up to 80 Percent of High-Risk Youth (January 7, 2008)
- Real-World Outcomes in Schizophrenia Are Focus of Two New NIMH Grants (January 4, 2008)
- Study Aims to Develop First Medications for Fragile-X Syndrome, Leading Inherited Cause of Mental Retardation (December 20, 2007)
- Depression Linked to Bone-Thinning in Premenopausal Women (November 26, 2007)
- Preschoolers with Three or More Coexisting Disorders Show No Response to ADHD Medication Treatment (November 5, 2007)
Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out:
- Press releases — promoted through distribution to major media; posted on the NIMH Web site
- Science updates — highlight recently published findings; also posted on the NIMH Web site
These are all also distributed to the public through the NIMH ListServ, which now has more than 20,000 subscribers.
If you have a manuscript accepted for publication that describes an especially significant finding, please contact your NIMH program director to discuss the possibility of a news release or other forms of dissemination.