Peter D. Kwong, Ph.D.
Structural Biology Section
Description of Research Program
The Structural Biology Laboratory seeks to apply structural biology to the development of an effective HIV-1 vaccine. Despite the enormous potential of atomic-level design—successfully used, for example, in the development of potent drugs against the HIV-1 protease—current vaccine development makes little use of atomic-level information. We are trying to change this.
|
|
|
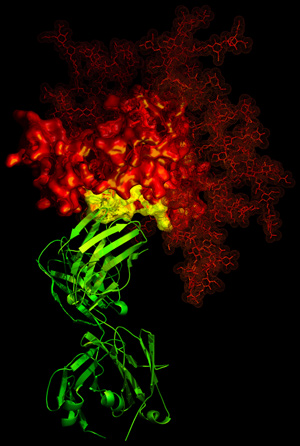
HIV-1 generally manages to evade antibody neutralization. Its gp120 glycoprotein (red) has one potential weakness: the site of binding (yellow) for the CD4 receptor. The b12 antibody (green) exploits this weakness—utilizing a functional requirement for rapid association with CD4—to neutralize HIV-1. This image, from X-ray crystallography, catches the b12 antibody in the act of grabbing onto this site of vulnerability (NIH news release). |
One area in which we and others have already made an impact is in understanding how HIV-1 is able to evade the humoral immune system. Determination of the structure of the HIV-1 gp120 envelope glycoprotein, the primary target of neutralizing antibodies against HIV-1, showed how N-linked carbohydrate can form both an immunologically silent face—with carbohydrate masquerading as "self"—and also can protect neighboring epitopes through an "evolving glycan shield" (1,2,18). We also showed how conformational flexibility of gp120 can combine with quaternary restrictions within the viral spike to prevent antibody neutralization (16). These and other studies, defining for example protective variable loops, have led to an understanding of the molecular trickery that protects HIV-1 from the humoral immune response.
But can one use structural biology in vaccine design? Currently, we are following two lines of investigation.
One line involves the precise delineation of functional constraints to identify potential footholds of conservation and exposure. Antibodies that bind to the co-receptor binding site on gp120 are capable of recognizing diverse strains of not only HIV-1, but also the more evolutionarily divergent HIV-2 (29). Such CD4i antibodies develop to high titers in most HIV-1 infected individuals. Unfortunately, the virus hides the site of co-receptor binding so that—prior to engagement of the primary HIV-1 receptor, CD4—the co-receptor site is not formed (21).
These studies demonstrate the strength of functional constraints in restricting epitope variation. But they also identify an important weakness: Functional conservation does not necessarily engender epitope exposure, which is required for antibody neutralization. We are currently exploring how function might constrain the site of CD4 binding, which—unlike the co-receptor-binding site—must be available as an initial site of attachment (32).
A second line of investigation involves structural analysis of the few broadly neutralizing antibodies that have been identified and that have the ability to neutralize diverse isolates of primary HIV-1. Only four antibodies of such ability have thus far been identified: the antibodies 2F5, 2G12, 4E10, and b12.
We have determined the structures of both 2F5 and b12, each with their HIV-1 envelope epitopes (27,32). In a collaborative study, primarily with Bill Schief’s and David Baker's groups at the University of Washington, Joe Sodroski's group at the Dana-Farber Cancer Institute, and also with Gary Nabel's and Rich Wyatt's groups at the Vaccine Research Center, we are currently generating epitope mimics, unencumbered by known mechanisms of humoral evasion. Tests of these epitope mimics in small animals should reveal their potential to elicit antibodies similar to the template broadly neutralizing ones.
Whether the confluence of structural information that we are generating is sufficient to elicit broadly neutralizing antibodies will depend in part on our ability to iteratively optimize immunogenicity and also on structural parameters of conformational mimicry, epitope accessibility, elicited potency, neutralization breadth, and target specificity. Our investigations have already led to insight into the parameters governing antibody elicitation and neutralization. True success, however, will depend on whether or not we succeed in creating immunogens capable of substantially reducing the incidence of HIV-1 infection in humans.
SBS Group Members (2008)
Research: Priyamvada Acharya, Ph.D.; Lei Chen, Ph.D.; Albert Kim, B.S.; Leo Kong, B.A.; Young Do Kwon, Ph.D.; Shahzad Majeed, M.S.; Jason McLellan, Ph.D.; Gilad Ofek, Ph.D.; Marie Pancera, Ph.D.; Mallika Sastry, Ph.D.; Anita Shah, Ph.D.; Tongqing Zhou, Ph.D.
Adjunct: Jeffrey Boyington, Ph.D.; Chantelle Hood, Ph.D.
Support: Jennifer Burke, executive assistant; Jonathan Stuckey, graphic artist

Links
Description of Research Program (2001)
Selected Publications
44. Davis, K. L., Bibollet-Ruche, F., Li, H., Decker, J. M., Kutsch, O., Morris, L., Salomon, A., Pinter, A., Hoxie, J. A., Hahn, B. H., Kwong, P. D., and Shaw, G. M. (2009). Human Immunodeficiency Virus Type 2 (HIV-2)/HIV-1 Envelope Chimeras Detect High Titers of Broadly Reactive HIV-1 V3-Specific Antibodies in Human Plasma. J. Virol. 83, 1240-1259.
43. Mörner, A., Douagi, I., Forsell, M. N., Sundling, C., Dosenovic, P., O'Dell, S., Dey, B., Kwong, P. D., Voss, G., Thorstensson, R., Mascola, J. R., Wyatt, R. T., Karlsson Hedestam, G. B. (2009). Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J. Virol. 83, 540-551.
42. Lam, S. N., Acharya, P., Wyatt, R., Kwong, P. D., and Bewley, C. A. (2008). Tyrosine-sulfate isosteres of CCR5 N-terminus as tools for studying HIV-1 entry. Bioorg .Med. Chem. 16, 10113–10120.
41. Madani, N., Schön, A., Princiotto, A. M., Lalonde, J. M., Courter, J. R., Soeta, T., Ng, D., Wang, L., Brower, E. T., Xiang, S. H., Kwon, Y. D., Huang, C. C., Wyatt, R., Kwong, P. D., Freire, E., Smith, A. B. 3rd, and Sodroski, J. (2008). Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 1689-1701.
40. Stricher, F., Huang, C. C., Descours, A., Duquesnoy, S., Combes, O., Decker, J. M., Kwon, Y. D., Lusso, P., Shaw, G. M., Vita, C., Kwong, P. D., and Martin, L. (2008). Combinatorial optimization of a CD4-mimetic miniprotein and cocrystal structures with HIV-1 gp120 envelope glycoprotein. J. Mol. Biol. 382, 510-524. PDB: 2i5y, 2i60.
39. Huang, C., Lam, S., Acharya, P., Tang, M., Xiang, S. H., Shahzad-ul-Hussan, S., Stanfield, R.L., Robinson, J., Sodroski, J., Wilson, I. A., Wyatt, R., Bewley, C. A., and Kwong, P. D. (2007). Structures of the CCR5 N-terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317, 1930-1934. PDB: 2rll, 2qad, CCR5Nt-docked (Zip).
38. Xie, H., Ng, D., Savinov, S. N., Dey, B., Kwong, P.D., Wyatt, R., Smith III, A. B., and Hendrickson, W. A. (2007). Structure-activity relations in the binding of chemically derived CD4 to human immunodeficiency virus gp120. J. Med. Chem. 50, 4898-4908.
37. Lin, G., Bertolotti-Ciarlet, A., Haggarty, B., Romano, J., McGeehan, K., Leslie, G., Polichiani d’Oliviere, A., Huang, C., Kwong, P. D., Doms, R. W., and Hoxie, J. A. (2007). Replication competent variants of human immunodeficiency virus type 2 lacking the V3 loop exhibit resistance to chemokine receptor antagonists. J. Virol. 81, 9956-9966.
36. Dey, B., Pancera, M., Svehla, K., Shu, Y., Xiang, S. H., Vainshtein, J., Li, Y., Sodroski, J., Kwong, P. D., Mascola, J. R., and Wyatt, R. (2007). Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: Antigenicity, biophysics, and immunogenicity. J. Virol. 81, 5579-5593.
35. Zhou, T., Xu, L., Dey, B., Hessell, A. J., Ryk, D. V., Xiang, S. H., Yang, X., Zhang, M. Y., Zwick, M. B., Arthos, J., Burton, D. R., Dimitrov, D. S., Sodroski, J., Wyatt, R., Nabel, G. J., and Kwong, P. D. (2007). Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445, 732-737. PDB: 2nxy, 2nxz, 2ny0, 2ny1, 2ny2, 2ny3, 2ny4, 2ny5, 2ny6, 2ny7.
34. Douek, D. C., Kwong, P. D., and Nabel, G. J. (2006). The rational design of an AIDS vaccine. Cell 124, 677-81.
33. Huang, C. C., Tang, M., Zhang, M. Y., Majeed, S., Montabana, E., Stanfield, R. L., Dimitrov, D. S., Korber, B., Sodroski, J., Wilson, I. A., Wyatt, R., and Kwong, P. D. (2005). Structure of a V3-containing HIV-1 gp120 core. Science 310, 1025-8. PDB: 2b4c.
32. Zhou, T., Hamer, D. H., Hendrickson, W. A., Sattentau, Q. J., and Kwong, P. D. (2005). Interfacial metal and antibody recognition. Proc Natl Acad Sci USA 102, 14575-80. PDB: 2adj, 2adi, 2adg.
31. Pancera, M., Lebowitz, J., Schon, A., Zhu, P., Freire, E., Kwong, P. D., Roux, K. H., Sodroski, J., and Wyatt, R. (2005). Soluble mimetics of human immunodeficiency virus type 1 viral spikes produced by replacement of the native trimerization domain with a heterologous trimerization motif: characterization and ligand binding analysis. J. Virol. 79, 9954-69.
30. Huang, C., Stricher, F., Martin, L., Decker, J. M., Majeed, S., Barthe, P., Hendrickson, W. A., Robinson, J., Roumestand, C., Sodroski, J., Wyatt, R., Shaw, G. M., Vita, C., and Kwong P. D. (2005). Scorpion-toxin mimics of CD4 in complex with human immunodeficiency virus gp120: Crystal structures, molecular mimicry, and neutralization breadth. Structure 5, 755-68. PDB: 1yyl, 1yym.
29. Decker, J. M., Bibollet-Ruche, F., Wei, X., Wang, S., Levy, D. N., Wang, W., Delaporte, E., Peeters, M., Derdeyn, C. A., Allen, S., Hunter, E., Saag, M. S., Hoxie, J. A., Hahn, B. H., Kwong, P. D., Robinson, J. E., and Shaw, G. M. (2005). Antigenic conservation and immunogenicity of the HIV co-receptor binding site. J. Exp. Med. 9, 1407-19.
28. Kwong, P. D. (2005). Refolding the envelope. Nature, 433, 815-816.
27. Ofek, G., Tang, M., Sambor, A., Katinger, H., Mascola, J. R., Wyatt, R., and Kwong, P. D. (2004). Structure and mechanistic analysis of the anti-HIV-1 antibody 2F5 in complex with Its gp41 epitope. J. Virol., 78, 10724-10737. PDB: 1tjg, 1tjh, 1tji.
26. Huang, C. C., Venturi, M., Majeed, S., Moore, M. J., Phogat, S., Zhang, M. Y., Dimitrov, D. S., Hendrickson, W. A., Robinson, J., Sodroski, J., Wyatt, R., Choe, H., Farzan, M., and Kwong, P. D. (2004). Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci USA. 101, 2706-11. PDB: 1rz7, 1rz8, 1rzi, 1rzg, 1rzf, 1rzk, 1rzj.
25. Kwong, P. D. (2004). The 447-52D antibody: Hitting HIV-1 where its armor is thickest. Structure 12, 173-4.
24. Burton, D. R., Desrosiers, R. C., Doms, R. W., Koff, W. C., Kwong, P. D., Moore, J. P., Nabel, G. J., Sodroski, J., Wilson, I. A., and Wyatt, R. T. (2004). HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 3, 233-6.
23. Xiang, S.-H., Wang, L., Abreu, M., Huang, C., Kwong, P. D., Rosenberg, E., Robinson, J. E., and Sodroski, J. (2003). Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 325, 124-134.
22. Kock, M., Pancera, M., Kwong, P. D., Kolchinsky, P., Grundner, C., Wang, L., Hendrickson, W. A., Sodroski, J., and Wyatt, R. (2003). Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology 313, 387-400.
21. Labrijn, A. F., Poignard, P., Raja, A., Zwick, M. B., Delgado, K., Franti, M., Binley, J., Vivona, V., Grundner, C., Huang, C. C., Venturi, M., Petropoulos, C. J., Wrin, T., Dimitrov, D. S., Robinson, J., Kwong, P. D., Wyatt, R. T., Sodroski, J., and Burton, D. R. (2003). Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77, 10557-10565.
20. Majeed, S., Ofek, G., Belachew, A., Huang, C., Zhou, T., and Kwong, P. D. (2003). Enhancing protein crystallization through precipitant synergy. Structure 11, 1061-1070. PDB: 1ps5.
19. Choe, H., Li, W., Wright, P. L., Vasilieva, N., Venturi, M., Huang, C., Grundner, C., Zwick, M. B., Wang, L., Rosenberg, E. S., Kwong, P. D., Burton, D. R., Robinson, J. E., Sodroski, J. G., and Farzan, M. (2003). Tyronsine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell 114, 161-170.
18. Wei, X., Decker, J. M., Wang, S., Hui, H., Kappes, J. C., Wu, X., Salazar-Gonzalez, J. F., Salazar, M. G., Kilby, J. M., Saag, M. S., Komarova, N. L., Nowak, M. A., Hahn, B. H., Kwong, P. D., and Shaw, G. M. (2003). Antibody neutralization and escape by HIV-1. Nature 422, 307-312.
17. Raja, A., Venturi, M., Kwong, P., and Sodroski, J. (2003). CD4 binding site antibodies inhibit human immunodeficiency virus gp120 envelope glycoprotein interaction with CCR5. J. Virol. 77, 713-718.
16. Kwong, P. D., Doyle, M. L., Casper, D. J., Cicala, C., Leavitt, S. A., Majeed, S., Steenbeke, T. D., Venturi, M., Chaiken, I., Fung, M., Katinger, H., Parren, P. W., Robinson, J., Van Ryk, D., Wang, L., Burton, D. R., Freire, E., Wyatt, R., Sodroski, J., Hendrickson, W. A., and Arthos, J. (2002). HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420, 678-682.
15. Xiang, S. H., Kwong, P. D., Gupta, R., Rizzuto, C. D., Casper, D. J., Wyatt, R., Wang, L., Hendrickson, W. A., Doyle, M. L., and Sodroski J. (2002). Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76, 9888-9899.
14. Sanders, R. W., Venturi, M., Schiffner, L., Kalyanaraman, R., Katinger, H., Lloyd, K. O., Kwong, P. D., and Moore, J. P. (2002). The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76, 7293-7305.
13. Yang, X., Lee, J., Mahony, E. M., Kwong, P. D., Wyatt, R., and Sodroski, J. (2002). Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76, 4634-4642.
12. Kwong, P. D., Wyatt, R., Majeed, S., Robinson, J., Sweet, R. W., Sodroski, J., and Hendrickson, W. A. (2000). Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure 8, 1329-1339. PDB: 1g9m, 1g9n.
11. Myszka, D. G., Sweet, R. W., Hensley, P., Brigham-Burke, M., Kwong, P. D., Hendrickson, W. A., Wyatt, R., Sodroski, J., and Doyle, M. L. (2000). Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 97, 9026-9031.
10. Yang, X., Florin, L., Farzan, M., Kolchinsky, P. Kwong, P. D., Sodroski, J., and Wyatt, R. (2000). Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74, 4746-4754.
9. Kwong, P. D., Wyatt, R., Sattentau, Q. J., Sodroski, J., and Hendrickson, W. A. (2000). Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of the human immunodeficiecy virus (HIV). J. Virol. 74, 1961-1972. PDB: Trimeric gp120 IRZJ (Zip), Trimeric gp120 HA proportionate (Zip).
8. Moulard, M., Lortat-Jacob, H., Mondor, I., Guillaume, R., Wyatt, R., Sodroski, J., Zhao, L., Olson, W., Kwong, P. D., and Sattentau, Q. J. (2000). Selective polyanion interactions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74, 1948-1960.
7. Kwong, P. D., Wyatt, R., Desjardins, E., Robinson, J., Culp, J. S., Hellmig, B. D., Sweet, R. W., Sodroski, J., and Hendrickson, W. A. (1999). Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1). J. Biol. Chem. 274, 4115-4123.
6. Kwong, P. D., and Liu, Y. (1999). Use of cryoprotectants in combination with immiscible oils for flash-cooling macromolecular crystals. J. Applied Crystallography 32, 102-105.
5. Zhang, W., Canziani, G., Plugariu, C., Wyatt, R., Sodroski, J., Sweet, R. W., Kwong, P. D., Hendrickson, W. A., and Chaiken, I. (1999). Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry 38, 9405-9416.
4. Binley, J. M., Wyatt, R., Desjardins, E., Kwong, P. D., Hendrickson, W. A., Moore, J. P., and Sodroski, J. (1998). Analysis of the interaction of antibodies with a conserved enzymatically deglycosylated core of the HIV type 1 envelope glycoprotein 120. AIDS Research Human Retroviruses 14, 191-198.
3. Rizzuto, C. D., Wyatt, R., Hernandez-Ramos, N., Sun, Y., Kwong, P. D., Hendrickson, W. A., and Sodroski, J. (1998). A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280, 1949-1953.
2. Wyatt, R., Kwong, P. D., Desjardins, E., Sweet, R. W., Robinson, J., Hendrickson, W. A., and Sodroski, J. (1998). The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393, 705-711.
1. Kwong, P. D., Wyatt, R., Robinson, J., Sweet, R. W., Sodroski, J., and Hendrickson, W. A. (1998). Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648-659. PDB: 1gc1.
back to top