 |
|
|
Topics on this Page
|
|
|
|
|
Background
FDA is working to address human infection with the 2009 H1N1 flu virus as part of a team led by the Department of Health and Human Services.
FDA is responding to this threat by:
- working with other government agencies and manufacturers on a series of issues related to antiviral medications
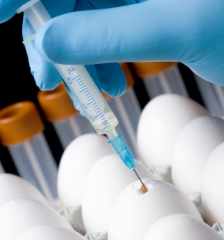
- growing the 2009 H1N1 flu virus and preparing to make vaccine seed lots, which may be used eventually to produce a safe and effective vaccine
- helping to prepare reagents needed for vaccine production and coordinating closely with other public health agencies for clinical development and testing
- accelerating access to new diagnostic tools for this 2009 H1N1 flu virus
To provide the latest information, FDA will update this Web page regularly.
Back to Top of Page
News
Back to Top of Page
Frequently Asked Questions
Back to Top of Page
Health Fraud
The U.S. Food and Drug Administration is alerting the public to be wary of Internet sites selling products that claim to prevent, treat or cure 2009 H1N1 flu virus, and is informing offending websites that they must take prompt action to correct and/or remove promotions of these fraudulent products or face immediate enforcement action.
Information for Consumers
FDA Regulated Products
- Drug Treatments for Flu
Tamiflu and Relenza are the two FDA-approved influenza antiviral drugs that are recommended by CDC for use against the 2009 H1N1 influenza virus. EUAs have been issued for specific uses of these two drugs in the current 2009 H1N1 setting.
- Personal Protective Equipment (PPE)
Information about masks, gloves, and gowns used to prevent the spread of germs from one person to another
- Test Kits
Information about potential risks and benefits of the H1N1 Flu Test Kit
- Biologics: Vaccines and Blood Safety
CBER's Vaccine and Blood Teams are working to facilitate the availability of safe and effective vaccines and ensuring that the blood supply is not affected by the H1N1 Flu Virus.
Back to Top of Page
Information
for Health Care Professionals
FDA Regulated Products
- Drug Treatments for Flu
Tamiflu and Relenza are the two FDA-approved influenza antiviral drugs that are recommended by CDC for use against the 2009 H1N1 influenza virus. EUAs have been issued for specific uses of these two drugs in the current 2009 H1N1 setting.
- Personal Protective Equipment (PPE)
Information about masks, gloves, and gowns used to prevent the spread of germs from one person to another.
- Medical Devices for Flu Diagnosis and Protection
Information about H1N1 influenza test kits and N95 respirators. FDA issued emergency use authorizations for some of these products in late April and early May.
- Biologics: Vaccines and Blood Safety
CBER's Vaccine and Blood Teams are working to facilitate the availability of safe and effective vaccines and ensuring that the blood supply is not affected by the H1N1 Flu Virus.
More Information
Back to Top of Page
Information
for Industry
Back to Top of Page |
 |
|
|
 |
 |
![]() Report Suspected Fraudulent Products or Criminal Activity Associated with H1N1 Flu Virus
Report Suspected Fraudulent Products or Criminal Activity Associated with H1N1 Flu Virus

