 |

Virtual Colonoscopy Identifies Large Polyps
Results from the American College of Radiology Imaging Network (ACRIN) National CT Colonography Trial, published in the September 18 New England Journal of Medicine, show that computed tomography (CT) colonography - also known as virtual colonoscopy - can detect 90 percent of adenomas (noncancerous tumors that can progress to cancer) or colorectal cancers measuring 1 centimeter or more in diameter.
These results compare favorably with standard optical colonoscopy, "which misses roughly 8 to 10 percent" of lesions of this size, says Dr. Carl Jaffe, chief of the NCI Cancer Imaging Program's Diagnostic Imaging Branch.
Read more 



Extra Copies of Chromosome Affects HER2 Test Results
Women with invasive breast cancer whose tumor cells have extra copies of chromosome 17, where the HER2 gene resides, are more likely to have borderline or "equivocal" results on HER2 testing, a team of Belgian researchers reports. In a study released September 15 in the Journal of Clinical Oncology (JCO), the researchers found that tumors with extra copies of chromosome 17 (a condition called polysomy 17) but not HER2 amplification - that is, extra copies of the HER2 gene - behaved like HER2-negative tumors.
The results of HER2 tests have significant implications for breast cancer patients, including whether they are candidates for the drug trastuzumab (Herceptin), which was specifically developed to treat HER2-positive disease.
Read more 
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Virtual Colonoscopy Identifies Large Polyps
 |
 |
Also in the Journals |
 |
| A supplement on "Improving Delivery of Colorectal Cancer Screening in Primary Care Practice" was recently published by the journal Medical Care. The supplement consists of 22 papers from a program of research sponsored by NCI and the Agency for Healthcare Research and Quality (AHRQ) to improve the delivery, utilization, and outcomes of colorectal cancer screening in primary care practice. The supplement presents approaches to delivering colorectal cancer screening in primary care settings and evaluating screening outcomes. This initiative directly addresses efforts to improve colorectal cancer screening uptake in the United States, which remains low - at about 50 percent. |
|
Results from the American College of Radiology Imaging Network (ACRIN) National CT Colonography Trial, published in the September 18 New England Journal of Medicine, show that computed tomography (CT) colonography - also known as virtual colonoscopy - can detect 90 percent of adenomas (noncancerous tumors that can progress to cancer) or colorectal cancers measuring 1 centimeter or more in diameter.
These results compare favorably with standard optical colonoscopy, "which misses roughly 8 to 10 percent" of lesions of this size, says Dr. Carl Jaffe, chief of the NCI Cancer Imaging Program's Diagnostic Imaging Branch.
Both CT colonography and colonoscopy can be employed to screen for precancerous polyps, the removal of which helps prevent the development of colorectal cancer. While colonoscopy uses a thin, tube-like instrument to physically examine the inside of the colon and rectum, CT colonography takes 2- or 3-dimentional pictures of the colon and rectum using a high-powered x-ray machine linked to a computer. The computed tomography technology required for CT colonography is already found in almost all hospitals, explains Dr. Jaffe.
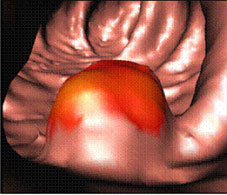

Virtual colonoscopy image of the inside of a colon. The red colored area indicates a polyp. Image courtesy of Dr. Ronald M. Summers, Diagnostic Radiology Department, Clinical Center, National Institutes of Health.
|  |
 |
"The less invasive nature of CT colonography and the low risk of procedure-related complications, as compared to colonoscopy, may be attractive to patients and may improve screening-adherence rates by addressing certain concerns of both patients and providers," state the authors.
Investigators at 15 hospitals participated in the ACRIN trial and enrolled more than 2,500 participants aged 50 or older who were scheduled for a screening colonoscopy. Participants first underwent CT colonography followed by standard colonoscopy, which was performed on the same day for 99 percent of participants.
The investigators compared results from the CT colonography exam to colonoscopy results for each patient, for the detection of lesions 5 millimeters or more in diameter, and calculated the false negative and false positive rates for CT colonography - the likelihood of missing a lesion (false negative) or falsely identifying a lesion that could not be found on follow-up colonoscopy (false positive).
While CT colonography could correctly identify 90 percent of people who had at least one polyp 10 millimeters in diameter or greater, the ability to correctly identify people who had smaller polyps was lower - down to 65 percent for polyps 5 millimeters in diameter.
 |
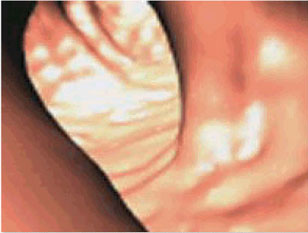

A still frame of an NIH Clinical Center video, which shows a virtual colonoscopy of the rectosigmoid colon performed in a retrograde fashion. The entire video can be viewed at http://www.cc.nih.gov/drd/colonoscopy.html on the NIH Clinical Center's Web site.
|
 |
The significance of these smaller lesions remains under debate. "Traditionally we've said that advanced adenomas are the ones with the most potential for developing into cancer," says Dr. Barry Kramer, associate director for disease prevention at the National Institutes of Health. The risk posed by smaller polyps "is the nub of a debate that's already started and will continue between radiologists and gastroenterologists," he explains.
Some additional questions about CT colonography remain to be answered, explains Dr. Robert Fletcher, professor emeritus from Harvard Medical School, in an accompanying editorial; in particular, whether CT colongraphy may miss some flat or depressed adenomas and what cumulative radiation dose may result from exposure to regular CT scans for screening.
The difficulty with flat and depressed adenomas, says Dr. Kramer, is that "we don't really yet know their natural history. We don't know how important it is to catch every last one," he explains. Both CT colonography and colonoscopy techniques need to be refined to better detect this type of adenoma.
Dr. Jaffe does not think that the radiation exposure, which is less than a standard CT scan, will be a large concern in this population. "For people over 50, the risk becomes diminutive, and if you use newer CT techniques, you can keep the exposure relatively low," he says.
For him, the most exciting thing about CT colonography is the ability to potentially reserve colonoscopy - which is more expensive and invasive, and currently requires specialists who are in short supply in the United States - for patients who have identified polyps, "so that [gastroenterologists] can concentrate on cases that merit polyp removal," he concludes.
—Sharon Reynolds
The current U.S. Preventive Services Task Force guidelines on colorectal cancer screening are available online at http://www.ahrq.gov/clinic/uspstf/uspscolo.htm.
|
 |
 |
Extra Copies of Chromosome Affects HER2 Test Results
Women with invasive breast cancer whose tumor cells have extra copies of chromosome 17, where the HER2 gene resides, are more likely to have borderline or "equivocal" results on HER2 testing, a team of Belgian researchers reports. In a study released September 15 in the Journal of Clinical Oncology (JCO), the researchers found that tumors with extra copies of chromosome 17 (a condition called polysomy 17) but not HER2 amplification - that is, extra copies of the HER2 gene - behaved like HER2-negative tumors.
The results of HER2 tests have significant implications for breast cancer patients, including whether they are candidates for the drug trastuzumab (Herceptin), which was specifically developed to treat HER2-positive disease.
"Because polysomy 17 on its own is not associated with HER2 overexpression and because it does not have the same clinicopathologic significance as true HER2 gene amplification, one may wonder whether polysomy 17 tumors benefit from HER2-targeted therapy such as trastuzumab," wrote Dr. Isabelle Vanden Bempt and colleagues from the University Hospital Gasthuisberg in Leuven, Belgium.
The researchers tested tumor samples from 226 patients using the two most common techniques for detecting HER2 - FISH and IHC. They also tested the samples for polysomy 17, finding that it was present in every patient with an equivocal HER2 result (47 of 226 patients), and that it was not associated with characteristics of HER2-positive cancers, such as hormone-receptor negativity or lower disease-free survival.
The authors called for clinical trials to help determine whether women with polysomy 17 tumors can benefit from trastuzumab treatment. In a related editorial in JCO, Dr. Carol Rosenberg from Boston University Medical Center, who lauded the current study, argued that new trials might be premature.
"This finding should influence the interpretation of HER2 test results and could eventually improve selection of patients for HER2-targeted treatment," she wrote. However, "New trials to directly test this question may not be the most efficient approach," she added, noting that review or reanalysis of data from existing trials could determine whether polysomy 17 tumors that lack HER2 are responsive to trastuzumab or lapatinib.
Vaccine Prevents HER2 Tumors in Mice
Researchers at the Karmanos Cancer Institute in Michigan have tested a vaccine that they found to be 100 percent effective at preventing tumors in mice injected with breast cancer cells. The results of their study appeared September 15 in Cancer Research.
The researchers used breast cancer cells that mimic the HER2-positive tumors found in women, which account for 20 to 30 percent of breast cancer cases. Women with HER2-positive tumors can be treated with drugs that target the HER2 receptor, such as trastuzumab (Herceptin) and lapatinib, but in some women these drugs eventually stop working.
The researchers used a panel of four cell types that overexpress HER2 and represent the various prognoses for women with HER2-positive breast cancer: two types that were completely sensitive to targeted drugs, one with initial sensitivity to targeted drugs but eventual resistance, and one with complete resistance to HER2-targeted drugs.
Mice were vaccinated with bacterial DNA engineered to include the gene sequence for a large portion of the HER2 receptor. The researchers used "electrovaccination," where an electric pulse encourages cells to absorb DNA and produce the related protein for presentation to immune cells.
After electrovaccination, the mice were injected with one of the four HER2 breast cancer lines. None of them developed tumors. However, control mice that had been electrovaccinated with a plasmid missing the HER2 DNA sequence developed tumors in every case. After 1 year of follow up, there were no adverse effects from vaccination.
In explaining the mechanisms of resistance to HER2-targeted drugs, the researchers noted that the various cell types they used in the study could co-exist in a single breast tumor, and that selective pressure after eliminating the drug-sensitive cells could cause increased growth of the drug-resistant cells. This would explain the relapse seen in some women.
Other tests in this study revealed aspects of how treatment-resistant cells in the mice were able to abandon HER2 and modify their cell-signaling strategy, employing an "escape mechanism" from HER2-targeted treatment.
"In patients whose tumors are refractory to drug and antibody therapy," the authors stated, "induction of comprehensive immunity by active vaccination will be critical to their long term protection."
Genetic Factors Tied to Blood Cancer Risk
It is still largely unknown what causes the group of diseases called myeloproliferative neoplasms (MPNs), including some rare forms of leukemia, where stem cells that replenish the blood supply begin to grow or differentiate out of control. But, a recent study shows their incidence is five to seven times higher among first-degree relatives of individuals who have been diagnosed with such a disease, providing strong evidence for a genetic link. This analysis, led by researchers from NCI's Division of Cancer Epidemiology and Genetics and collaborators in Sweden, appeared September 15 in Blood.
Using population-based data from Sweden, which has a universal health care system, the researchers identified all patients who had been diagnosed with an MPN between 1958 and 2005. For each patient, four population-based controls matched by sex, year of birth, and county of residence were chosen randomly from the Swedish Population database. From the Swedish Multigenerational Registry, which includes information on parent-offspring relations for all Swedish citizens who were born in 1932 or later, the researchers obtained information on all first-degree relatives (parents, siblings, and offspring) of both patients and controls and linked them to the Swedish Cancer Registry to collect information on incident cancer cases. The study included a total of 11,039 patients with an MPN, 43,550 controls, 24,577 first-degree relatives of patients, and 99,542 first-degree relatives of controls.
The researchers found that the relative risk of developing an MPN among first-degree relatives of patients (versus first-degree relatives of controls) was increased 5.6-fold. The relative risk for specific diseases including polycythemia vera (PV), essential thrombocythemia (ET), and myeloproliferative neoplasm unclassifiable (NOS) was 5.7, 7.4, and 7.5, respectively. Only myelofibrosis (MF) - a disease in which the bone marrow is replaced by fibrous scar tissue - showed no increased risk among first-degree relatives of patients, though the overall incidence of this disease was very rare. Compared to first-degree relatives of controls, first-degree relatives of patients with MPNs had a borderline-significant 1.9-fold increased risk of chronic myeloid leukemia.
"Our results support the theory that there are common, strong, shared susceptibility genes that predispose to PV, ET, MF, and possibly chronic myeloid leukemia," the researchers conclude, noting that this is the largest population-based, case-control study of its kind, and that it "supports the application of gene mapping and candidate gene approaches in high-risk families and case-control studies."
In a related comment in the journal, Dr. Jerry Spivak of Johns Hopkins University concludes that the present study serves to remind us that in MPNs, disease characteristics are "driven as much and possibly more by as-yet-undefined genetic influences as it is by those that have been defined."
Most Cancer Clinical Trials Go Unpublished
Findings from fewer than one in five registered cancer clinical trials are published in peer-reviewed journals, according to a study that appeared September 15 in The Oncologist. This finding, the authors state, raises the concern of publication bias in cancer clinical trials.
Drs. Scott Ramsey and John Scoggins of the Fred Hutchinson Cancer Research Center and the University of Washington found that between 1999 and 2007, only 17.6 percent of cancer-related trials registered with ClinicalTrials.gov (the federal registry for clinical trials of interventions for serious or life-threatening conditions) went on to be published in widely accessible journals listed in the PubMed.gov online database.
The researchers also found evidence of a selection bias on the part of investigators, who are less likely to publish results of a trial that did not meet its endpoints. Though more than 94 percent of the registered, industry-sponsored trials were never published, three-fourths of those in PubMed.gov reported positive results. By comparison, 59 percent of NCI-supported trials were published, half of which reported negative results.
In a related commentary, Dr. James H. Doroshow, director of NCI's Division of Cancer Treatment and Diagnosis, wrote that "the apparent lack of access to the final efficacy and toxicity data…poses multiple scientific and ethical questions." He described an NCI clinical trials database project expected to launch in 2009 that will address this problem by requiring administrative and outcome data for all intervention studies that receive NCI support.
The Oncologist is also considering creating a peer-reviewed, searchable venue for "well-executed trials that fail to meet positive endpoints," wrote Editor-in-Chief Dr. Bruce A. Chabner and Senior Editor Dr. Gregory A. Curt, for trials that are "'negative' in a sense, but valuable nonetheless."
|
 |
 |

Guest Update by Dr. Robert Croyle
Understanding the Media's Power to Influence Tobacco Use and Control
 Despite progress made in reducing tobacco use, today fully one-third of the nation's cancer deaths are caused by cigarette smoking. Approximately 20 percent of American adults still smoke and more than 4,000 adolescents smoke their first cigarette each day. Despite progress made in reducing tobacco use, today fully one-third of the nation's cancer deaths are caused by cigarette smoking. Approximately 20 percent of American adults still smoke and more than 4,000 adolescents smoke their first cigarette each day.
Why are these rates still so high? Part of the answer is explained in the recently released 19th volume of the NCI Tobacco Control Monograph Series, The Role of the Media in Promoting and Reducing Tobacco Use, which was highlighted at a press conference sponsored by the American Legacy Foundation, the Campaign for Tobacco-Free Kids, the American Public Health Association, and the American Medical Association.
The report outlines six major conclusions, including the fact that tobacco advertising and promotion are causally related to increased tobacco use, and depictions of smoking in movies are causally related to youth smoking initiation. Given that cigarettes are one of the most heavily marketed products in the United States, and that depictions of smoking were found to occur in more than three-quarters of contemporary box-office hits, these findings are particularly disturbing.
The monograph, which took more than 5 years to complete, involved 5 scientific editors, 23 authors, and 62 expert reviewers, who analyzed more than 1,000 scientific studies on the role of media in encouraging and discouraging tobacco use. Research included in the monograph comes from the disciplines of marketing, psychology, communication, statistics, epidemiology, and public health - all vital to understanding how exposure to the media influences tobacco use.
And the evidence from that research is clear and convincing. The editors concluded, for example, that mass media campaigns to discourage tobacco use can change youth attitudes about tobacco, reduce the chances children will smoke, and encourage adult cessation. The effect on initiation appears greater in controlled field experiments when mass media campaigns are combined with school- and community-based programming. Many population studies document reductions in smoking prevalence when mass media campaigns are combined with other strategies in multi-component tobacco control programs.
Tobacco use still accounts for more than 400,000 premature deaths in the United States each year. Understanding the role of media in reducing or promoting tobacco use is of critical importance in fighting the tobacco epidemic. It is our hope that this new monograph will inform tobacco prevention and control research, practice, and policy for years to come in the United States and around the world. If it is successful in that regard, we know many lives will be saved.
Dr. Robert T. Croyle
Director, NCI Division of Cancer Control and Population Sciences |
 |
 |

NCI Expert Testifies on Biospecimen Policies
At a September 9 hearing chaired by Representative Brad Miller (D-NC), Dr. Jim Vaught, deputy director of NCI's Office of Biorepositories and Biospecimen Research (OBBR), testified before the U.S. House of Representatives Committee on Science and Technology, recommending best practices and policies for biospecimen storage and tracking.
Federal scientific collections are often subject to highly variable and substandard quality management practices, frequently without long-term plans for custodianship, Dr. Vaught stated. He also indicated that many of these collections are priceless and irreplaceable, and adopting practices such as those developed by NCI and other groups will be critical to preserving these collections in the condition necessary to make scientific discoveries and medical advances.
"We are mindful that when patients and other study participants agree to provide blood and other samples for research, they do so with an expectation that their tissue will be used to provide insight into the causes and cures of their disease, or to advance medical research in general," said Dr. Vaught.
To read Dr. Vaught's full testimony or learn more about NCI's initiatives to standardize biospecimen procedures to ensure the availability of high-quality human specimens for cancer research, visit http://biospecimens.cancer.gov.
|
 |
 |

FDA Approves Anti-nausea Patch, Expands Indication of HPV Vaccine
 |
 |
Support for Those Affected by Natural Disasters |
 |
Resources are available to those affected by the recent hurricanes on the Cancer.gov home page. Please click this tile for more information:
 |
|
A skin patch developed to prevent the nausea and vomiting often induced by chemotherapy was approved by the Food and Drug Administration (FDA) earlier this month. The patch, called Sancuso, is expected to be available to patients later this year.
Sancuso delivers granisetron, an established inhibitor of nausea and vomiting that is currently available in oral and intravenous forms. The patch was designed to release granisetron slowly into the patient's bloodstream for up to 5 consecutive days.
According to ProStrakan, the Scottish company that developed the patch, Sancuso was generally well-tolerated by patients in clinical trials. The most common drug-related adverse reaction was constipation. Skin reactions at the application site were reported but were mild and did not lead to discontinuation of use.
Earlier this month, the FDA also expanded its approval of the HPV vaccine Gardasil for the prevention of vaginal and vulvar cancers.
Manufactured by Merck & Co., Gardasil was first approved for women and girls aged 9 to 26 by the FDA in 2006 to prevent infection by HPV types 16, 18, 6, and 11. These four HPV types together cause 70 percent of cervical cancers, 90 percent of genital warts, and an unspecified percentage of vulvar and vaginal cancers.
After following 15,000 participants from the original studies for 2 additional years, researchers found that Gardasil was highly effective in preventing the types of precancerous vulvar and vaginal lesions caused by HPV. Among women who had not received the vaccine, 9 developed precancerous vaginal lesions and 10 developed precancerous vulvar lesions, whereas no such cases were found in the Gardasil group.
This is "strong evidence," said Dr. Jesse L. Goodman, director of the FDA's Center for Biologics Evaluation and Research. "While vulvar and vaginal cancers are rare, the opportunity to help prevent them is potentially an important additional benefit from immunization against HPV." |
 |
 |

Cell Phones and Brain Cancer: What We Know (and Don't Know)
Concerns about the potential health effects of using cellular telephones were back in the news this summer. But these concerns - and specifically the suggestion that using a cell phone may increase a person's risk of developing brain cancer - are not supported by a growing body of research on the subject.
More than a dozen studies have explored the relationship between the use of cell phones and malignant or benign brain tumors. The majority of these have found little or no overall increased risk of brain tumors within the first 10 years of use. Studies now in the pipeline will yield information on longer-term use, as well as the first results involving children.
"We now have studies covering up to 10 years of cell phone usage, and we're still not seeing any convincing evidence of an increased brain cancer risk," said Dr. Peter Inskip of NCI's Division of Cancer Epidemiology and Genetics, who led one of the first studies on the subject. He recently briefed NCI's National Cancer Advisory Board on what is known and not known about cell phones and cancer.
A major unanswered question is how cell phones might contribute to cancer. Cell phones emit radiofrequency energy, which is a form of electromagnetic radiation. While exposure to high levels of radio frequency energy can heat body tissues, the amount of radiofrequency energy produced by cell phones is too low to cause significant heating of tissue.
"The biological mechanism by which radiofrequency radiation might cause cancers is unknown and entirely a matter of speculation," said Dr. Inskip.
The controversy over cell phones and cancer started on national television in 1993, when Larry King interviewed a man who said that his wife's fatal brain tumor had been caused by her cell phone. The issue received considerable attention in the media, and cell phone stocks plummeted temporarily. Within a week, Congress had asked NCI and other agencies to investigate.
Dr. Inskip was at the time preparing a study to attempt to identify causes of brain cancer, and he added a component to assess cell phone usage. The results, reported in the New England Journal of Medicine in 2001, showed no evidence of an association between recent cell phone use and brain cancer. The conclusion was supported by two other reports published at about the same time.
Since then, most studies have not found a link between cell phones and cancer. The studies have been launched largely in response to people's concerns that any negative health effects from this new technology would be a major public health issue. In the United States alone, there were more than 255 million cell phone subscribers last year, up from 110 million users in 2000 and 208 million in 2005.
Given this dramatic growth, researchers have looked for increases in the incidence of brain cancer in the U.S. population and found none. There was no upturn in the incidence of brain or other nervous system cancers between 1987 and 2005, according to data from NCI's Surveillance, Epidemiology, and End Results program.
Another avenue of research has focused on people who were exposed to increased levels of radiofrequency energy in the workplace. Two such studies - one of employees in a factory manufacturing cell phones and the other of veteran Navy radar technicians exposed during the Korean War - showed no evidence of increased cancer risk.
Because cell phone technology is so new and has changed over time, larger studies are needed to answer questions about longer-term use. Answers may come from a series of multinational studies collectively known as the INTERPHONE study. While the combined analysis is not yet complete, some of the 13 participating countries have pooled their data and reported little or no effect on the risk of brain tumors.
Several European countries are also examining cell phone use in children and adolescents diagnosed with brain cancer. Children may be at greater risk of health effects than adults because their nervous systems are still developing at the time of exposure. In addition, young people may accumulate many years of exposure during their lifetimes.
For the future, it will be important to look for the consistency of results both within and across studies, said Dr. Inskip. "With more data and the ability to look at data in many different ways, some positive results are likely to occur simply by chance," he added.
Of all the potential health risks associated with cell phones that have been examined so far, the most convincing evidence concerns the risk of motor vehicle accidents among people distracted by using their cell phone while driving, Dr. Inskip noted.
—Edward R. Winstead
For a list of published studies and more information see NCI's fact sheet on Cellular Telephone Use and Cancer Risk.
|
 |
 |
 |

New Drug for Patients with Metastatic or Inoperable Kidney Cancer
Name of the Trial
Phase II Study of Vandetanib in Patients with Metastatic or Unresectable Clear Cell Renal Cell Carcinoma (NCI-08-C-0039). See the protocol summary at http://www.cancer.gov/clinicaltrials/NCI-08-C-0039.

Dr. W. Marston Linehan
|  |
Principal Investigators
Dr. W. Marston Linehan and Dr. Ramaprasad Srinivasan (Lead Investigator), NCI Center for Cancer Research
Why This Trial Is Important
Renal cell carcinoma (RCC), the most common type of kidney cancer, often stimulates the growth of a large supply of new blood vessels (angiogenesis) to provide the oxygen and nutrients needed for continued tumor growth. Two drugs recently approved by the U.S. Food and Drug Administration for the treatment of patients with RCC, sorafenib (Nexavar) and sunitinib (Sutent), work by disrupting the angiogenesis process. However, RCC tumors often develop resistance to these drugs.
A new drug called vandetanib (Zactima) also inhibits angiogenesis by interfering with a protein involved in the process: vascular endothelial growth factor receptor 2 (VEGFR2). In addition, vandetanib also inhibits epidermal growth factor receptor (EGFR), a protein that mediates several functions essential for tumor cell growth. Clear cell RCC "is characterized by mutations in a gene we identified that is called the VHL gene," explained Dr. Linehan. "VHL regulates a number of things, including angiogenesis. Vandetanib targets two important parts of the VHL gene pathway - VEGFR2 and EGFR."
In this randomized trial, patients who have RCC that cannot be surgically removed (unresectable) or that has spread (metastatic) and who have previously received sorafenib or sunitinib will take vandetanib daily until their disease progresses or they develop unacceptable side effects.
In addition to monitoring the patients' tumors, the researchers will perform magnetic resonance imaging scans to visualize how vandetanib affects the blood supply to the tumors. The researchers will also collect blood samples from all participants to see if vandetanib is affecting the targeted proteins in the VHL gene pathway.
"We spent 10 years identifying this gene here at NCI, so we're thrilled to be conducting this trial targeting the VHL pathway," said Dr. Linehan.
For More Information
See the lists of entry criteria and trial contact information at http://www.cancer.gov/clinicaltrials/NCI-08-C-0039 or call the NCI's Clinical Trials Referral Office at 1-888-NCI-1937. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
Patricia Steeg Receives 2008 Brinker Award
 Dr. Patricia Steeg, head of the Women's Cancers Section in NCI's Laboratory of Molecular Pharmacology, has been awarded the Komen Brinker Award for Scientific Distinction - the highest honor conferred by Susan G. Komen for the Cure. Dr. Steeg will receive the basic science award along with fellow clinical science awardees Dr. Richard Gelber of the Dana-Farber Cancer Institute and Dr. Aron Goldhirsch of the European Institute of Oncology. Dr. Patricia Steeg, head of the Women's Cancers Section in NCI's Laboratory of Molecular Pharmacology, has been awarded the Komen Brinker Award for Scientific Distinction - the highest honor conferred by Susan G. Komen for the Cure. Dr. Steeg will receive the basic science award along with fellow clinical science awardees Dr. Richard Gelber of the Dana-Farber Cancer Institute and Dr. Aron Goldhirsch of the European Institute of Oncology.
Dr. Steeg is being recognized for her work in tumor metastasis, identifying the first metastasis suppressor gene and bringing this research to clinical trial, as well as pioneering work on brain metastasis of breast cancer.
The Komen Brinker Award was established in 1992 to recognize leading scientists for their significant work in advancing research concepts or clinical application in the fields of breast cancer research, screening, or treatment. The recipients will receive their awards at a dinner presentation on December 11, and they are also invited to present their work in a lecture at the annual San Antonio Breast Cancer Symposium.
NCI Patient Education Materials Receive Awards
The Health Information Resource Center (HIRC) recently presented NCI's Office of Communications and Education with five National Health Information Awards. The HIRC, a national clearinghouse for consumer health information programs and materials, received more than 1,000 entries. The awards recognize the nation's best consumer health information programs and materials produced each year. Judging is based on content, format, success in reaching the targeted audience, and overall quality.
NCI's "Toolkit to Improve Health Literacy for Radiation Therapy Patients" (in English and Spanish) received a gold award; the Radiation Therapy Fact Sheet Series received a silver award; "La radioterapia y usted: Apoyo par alas personas con cancer" received a bronze award; and merit awards were given to "What You Need To Know About Thyroid Cancer," "You Can Manage Radiation Therapy Side Effects," and "Chemotherapy and You: Support for People with Cancer."
SBIR to Showcase Products and Research
NCI's Division of Cancer Control and Population Sciences is sponsoring the fifth annual Small Business Innovation Research (SBIR) showcase, "Linking eHealth Science and Business," on October 6-7, at the Hyatt Regency in Bethesda, MD. NCI-funded SBIR grantees will present user-centered, science-based, commercially viable products, and their final research findings to staff from several government institutes, the public health community, and commercial organizations.
Additional details about the event and the SBIR program can be found at: http://cancercontrol.cancer.gov/hcirb/sbir/research/2008_showcase.html.
New Clinical Trial Reporting Requirements Coming in 2009
Beginning next January, NCI grantees must adhere to a new set of mandatory clinical trial reporting requirements. This new Clinical Trials Reporting Program will include up-to-date information about the status of all NCI-funded and sponsored clinical research, patient demographics and accruals, treatment, and toxicity outcomes.
These new requirements will provide NCI with a more global view of emerging knowledge to identify important patterns in a timely way in order to assure patient safety and an optimal return on investment in cancer clinical trials. The data provided will allow NCI to better coordinate research efforts and ensure patient safety to optimize the investment in cancer research.
For more information and updates about these changes, go to www.cancer.gov/ncictrp.
Disparities Summit Report Available
 NCI's Center to Reduce Cancer Health Disparities, the National Center on Minority Health and Health Disparities, and the National Center for Research Resources recently released the report on the Cancer Health Disparities Summit 2007, Catalyzing Trans-disciplinary Regional Partnerships to Eliminate Cancer Health Disparities. The report evolved from a 3-day workshop held July 16-18, 2007 and can be accessed and downloaded at http://www.cancermeetings.org/chdsummit07/FinalReport.pdf. NCI's Center to Reduce Cancer Health Disparities, the National Center on Minority Health and Health Disparities, and the National Center for Research Resources recently released the report on the Cancer Health Disparities Summit 2007, Catalyzing Trans-disciplinary Regional Partnerships to Eliminate Cancer Health Disparities. The report evolved from a 3-day workshop held July 16-18, 2007 and can be accessed and downloaded at http://www.cancermeetings.org/chdsummit07/FinalReport.pdf.
| |
 |
 |

Dr. Ana Maria Lopez
Associate Professor of Clinical Medicine and Pathology, University of Arizona College of Medicine
 Walnut trees grow on hillsides and old Spanish missions dot the landscape around the Mariposa Community Health Center in Nogales, AZ. In this town of 24,000, 88 percent of the residents are Hispanic and many of them are underserved and poor, including Marietta, a 45-year-old woman who has cervical cancer. She is unable to travel to Tucson to see an oncologist because the family's pickup truck won't survive the trip, and her family simply cannot afford it. Walnut trees grow on hillsides and old Spanish missions dot the landscape around the Mariposa Community Health Center in Nogales, AZ. In this town of 24,000, 88 percent of the residents are Hispanic and many of them are underserved and poor, including Marietta, a 45-year-old woman who has cervical cancer. She is unable to travel to Tucson to see an oncologist because the family's pickup truck won't survive the trip, and her family simply cannot afford it.
But 65 miles away in Tucson, Dr. Ana Maria Lopez, an oncologist and medical director of the University of Arizona's Telemedicine Program, will see that Marietta receives state-of-the-art care without leaving Nogales. While Marietta's physician examines her cervix, Dr. Lopez will receive digital images of the procedure and work with cervical cancer specialists to determine Marietta's next treatment steps. This study, funded by NCI, is assessing the clinical- and cost-efficacy of telecolposcopy.
Under Dr. Lopez's leadership, the University of Arizona Telemedicine Program performs consults for as many as 2,000 patients a year, including members of Arizona's 28 Native American tribes. She is also the principal investigator of a 4-year American Cancer Society grant on "Examining Barriers to Minority Participation in Cancer Clinical Trials" to determine whether the beliefs of ethnically distinct populations affect the accrual of Latino/Hispanic participants to clinical trials. And, she has been funded on a number of NIH grants dealing with disparities and underserved populations.
"Dr. Lopez has brought the leading cancer center in Arizona to us," says Dr. Eladio Pereira, chief internist and medical director at the Mariposa Community Health Center. Currently, Dr. Lopez is using telemedicine to treat 50 of Dr. Pereira's patients and has set up a telemedicine program to treat and provide follow-up care to breast cancer survivors in Nogales.
Born in Bolivia, Dr. Lopez was only 6 years old when her physician parents immigrated to Chicago to complete their residency training in pathology. She studied philosophy, political science, and chemistry as an undergraduate, and it was during this time that her beliefs regarding equality and compassion in medicine began to take form.
She graduated from Jefferson Medical College and completed her residency and fellowship training in internal medicine, hematology, and oncology, at the University of Arizona in Tucson. Ten years later, she completed her master's degree in public health from the same institution and in 1995 she completed a post-doctoral NIH fellowship in cancer prevention and etiology. Dr. Lopez's interest in medical oncology emerged from her passion for clinical care.
"With the rapid developments in oncology," she says, "working with cancer patients allows me to practice state-of-the-art clinical care that addresses the patient's physical and emotional needs, while integrating the latest developments in translational medicine.
"To care for people is an incredible privilege," she added. "The secret is simply to care for your patient…if you keep that as your focus, you'll do the right thing. In medicine you are always learning and that is what keeps things exciting."
Her latest project is called Ultra Clinic. Normally, patients in rural towns like Nogales have to wait months for the results of a mammogram before knowing whether a biopsy should be performed. "We have integrated the process so the patient can get the results the same day via telemammography readings," Dr. Lopez says.
In July, she demonstrated the use of telemedicine and new Web-based technologies at the White House. The meeting was attended by medical directors from the White House, the U.S. State Department, the Central Intelligence Agency, the Federal Bureau of Investigation, and other federal agencies. In June, she made the cover of the magazine Tucson Lifestyle after being named one of the city's top doctors, as selected by her peers - an honor she has received for several years in a row.
—Francis X. Mahaney, Jr. |
 |
|