 |

Clinical Trial Endorses Chemotherapy Alone for
Early-Stage Lymphoma
A clinical trial comparing treatments for early stage aggressive lymphoma has found that an intensive regimen of chemotherapy is better than chemotherapy plus radiation for treating the disease in its early stages, according to a study in the March 24 New England Journal of Medicine.
Dr. Felix Reyes of the Hopital Henri Mondor in Creteil, France, and his colleagues found that an intensive regimen of doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) was superior to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) plus radiation.
"After a median follow-up of 7.7 years, we found superior event-free and overall survival rates among patients treated with chemotherapy alone," the researchers wrote.
The 5-year estimates of event-free survival were 82 percent for patients receiving chemotherapy alone and 74 percent for those receiving chemotherapy plus radiation. The
respective 5-year estimates of overall survival were 90 percent and 81 percent.
Read more 


Training Future Leaders, Ensuring Future Success
It's always rewarding to be recognized for a job well done, especially when you are so firmly committed to that job. So it's heartening to see that the National Cancer Institute's (NCI) campuses in Maryland have, for the third year in a row, been selected among the top institutions for postdoctoral life sciences researchers in the United States by readers of The Scientist magazine. The accolade is the result of voting by more than 3,500 postdoctoral fellows from the United States, Canada, and Europe based on criteria such as the value of the training they received, access to research equipment and library resources, and good mentoring relationships.
At the NCI Center for Cancer Research (CCR) Fellows and Young Investigators retreat last month, it was easy to see the results of this training in action. Clinical and research fellows, visiting scientists, postbaccalaureate fellows, and other young investigators from CCR heard talks from leading NCI researchers and investigators from the extramural community and shared results from their own exciting research - work that is at the heart of advances being made by NCI's intramural programs.
Read more 
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Clinical Trial Endorses Chemotherapy Alone for
Early-Stage Lymphoma
A clinical trial comparing treatments for early stage aggressive lymphoma has found that an intensive regimen of chemotherapy is better than chemotherapy plus radiation for treating the disease in its early stages, according to a study in the March 24 New England Journal of Medicine.
Dr. Felix Reyes of the Hopital Henri Mondor in Creteil, France, and his colleagues found that an intensive regimen of doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) was superior to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) plus radiation.
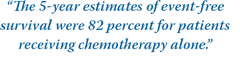 "After a median follow-up of 7.7 years, we found superior event-free and overall survival rates among patients treated with chemotherapy alone," the researchers wrote.
"After a median follow-up of 7.7 years, we found superior event-free and overall survival rates among patients treated with chemotherapy alone," the researchers wrote.
The 5-year estimates of event-free survival were 82 percent for patients receiving chemotherapy alone and 74 percent for those receiving chemotherapy plus radiation. The
respective 5-year estimates of overall survival were 90 percent and 81 percent.
The study included 647 previously untreated patients with localized stage I or II aggressive lymphoma. All were between the ages of 15 and 61, and the most common subtype in the group was diffuse large B-cell lymphoma.
"This study indicates that highly effective chemotherapy regimens such as ACVBP are more effective than the older CHOP chemotherapy plus radiation for these patients," comments Dr. Wyndham Wilson of the Lymphoma Section of NCI's Experimental Transplantation and Immunology Branch. "So if you have very active chemotherapy, you don't need radiation for the early stage of the disease."
One of the contributions of this study, he adds, is to illustrate the concept that "good chemotherapy is probably the best you can do for this disease."
Three cycles of CHOP followed by radiation has been considered the standard therapy for localized lymphoma since a 1998 study found it to be superior to CHOP alone in a randomized trial of 400 patients.
Developed by the Groupe d'Etude des Lymphomes de l'Adulte, ACVBP consists of an induction phase with higher doses of doxorubicin and cyclophosphamide than those used in CHOP and a consolidation phase consisting of treatment with the drugs not used during induction. The ACVBP regimen is less well tolerated in elderly patients.
The current research was undertaken based on a previous study of two chemotherapy regimens for intermediate or high-grade lymphoma. In that study, the estimated 5-year rate of overall survival among patients with localized disease who received the ACVBP regimen was 80 percent.
In an accompanying editorial, Dr. James Armitage of the University of Nebraska Medical Center in Omaha discusses efforts to improve the classification of patients with lymphomas based on distinctive biological characteristics, such as patterns of gene activity, that one day can be used to make decisions about treatments.
Dr. Armitage observes, for example, that ACVBP may not have been the best treatment for all patients in the trial. He concludes, "Physicians who treat patients with lymphomas hope that we will continue to move away from the 'one-size-fits-all' approach."
By Edward R. Winstead
|
 |
 |

Training Future Leaders, Ensuring Future Success
It's always rewarding to be recognized for a job well done, especially when you are so firmly committed to that job. So it's heartening to see that the National Cancer Institute's (NCI) campuses in Maryland have, for the third year in a row, been selected among the top institutions for postdoctoral life sciences researchers in the United States by readers of The Scientist magazine. The accolade is the result of voting by more than 3,500 postdoctoral fellows from the United States, Canada, and Europe based on criteria such as the value of the training they received, access to research equipment and library resources, and good mentoring relationships.
At the NCI Center for Cancer Research (CCR) Fellows and Young Investigators retreat last month, it was easy to see the results of this training in action. Clinical and research fellows, visiting scientists, postbaccalaureate fellows, and other young investigators from CCR heard talks from leading NCI researchers and investigators from the extramural community and shared results from their own exciting research - work that is at the heart of advances being made by NCI's intramural programs.
The attendees at this retreat, and their colleagues in cancer centers and other institutions across the country, are tomorrow's cancer research leaders. They will fulfill the promise of everything we are learning and achieving today.
NCI was one of the first National Institutes of Health institutes to establish an office dedicated to training young investigators. The CCR Office of Training and Education, led by Dr. Jonathan Wiest, helps to empower fellows by promoting and organizing training opportunities, implementing new courses and training programs that prepare fellows to become successful independent biomedical researchers, and providing funding mechanisms to reward outstanding research efforts by postdoctoral fellows.
NCI's Division of Cancer Epidemiology and Genetics' (DCEG) Office of Education and its chief, Dr. Demetrius Albanes, provide research training for the full range of cancer risk
factors, from nutrition to environmental exposures to infectious agents.
NCI is such a fertile training ground, in part because of its unique offerings. CCR, for example, has partnered with The Johns Hopkins University to create a new concentration in Hopkins' Master of Science in Biotechnology program called Molecular Targets and Drug Discovery Technologies. This innovative program will recruit immediate postbaccalaureates to work in CCR laboratories on projects related to discovering and developing molecular targets of cancer while they attend classes to earn their master's degrees. DCEG also is partnering with Hopkins and other universities to provide graduate education and research training in cancer epidemiology.
A number of innovative training courses are also offered at NCI, one of the most popular of which is Translational Research in Clinical Oncology (TRACO). This course delves into the general principles of cancer biology and treatment, epidemiology, mechanisms of resistance, metastasis, use of preclinical models, and identification of novel molecular targets. The TRACO course provides an unprecedented opportunity for less experienced researchers to glimpse the future of translational research in clinical oncology and meet leaders in cancer research.
Although we are making - and clearly must continue to make - tough choices about where to best allocate our dollars, I am committed to ensuring that we continue to nurture the careers of young investigators, including those in the NCI intramural program and in the extramural community. Achieving this end is inextricably linked to our 2015 goal.
Dr. Andrew C. von Eschenbach
Director, National Cancer Institute
|
 |
 |

RNAi and Cancer: Silencing Essential Genes
 |
 |
Administrative Supplements Available
Applications are being accepted for administrative supplements for NCI-funded cancer control intervention research R01, P01, P50, U01, and U19 grants. They are designed to provide 1-year funding to cancer control investigators whose intervention efficacy data have been analyzed and who are conducting peer-reviewed research with an active NCI grant award related to the intervention program proposed for dissemination.
Application receipt date is May 30, 2005. For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2647. Inquiries: Dr. Jon F. Kerner-
jon.kerner@nih.gov.
|
 |
|
|
The discovery that genes can be silenced by injecting short strands of RNA into cells - a process known as RNA interference or RNAi - has revolutionized biological research. Experiments that once took months or years are now done in days or weeks.
Most laboratories and drug companies working on cancer today use RNAi to identify cancer genes and to investigate the underlying biology of the disease.
"Cancer biologists are using RNAi to do everything from investigating individual genes to running high-throughput screens for new drugs to developing therapeutics," says Dr. Natasha Caplen, head of the Gene Silencing Section in NCI's CCR.
Her group is investigating the mechanics of RNAi in mammalian cells and studying 400 genes associated with cancer, among other projects. "We want to know how we can use this technology to better understand the biology of cancer and the differences between healthy cells and cancer cells," explains Dr. Caplen.
RNAi exploits a defense mechanism in cells that recognizes and degrades the RNA of invaders such as viruses. To turn this mechanism against a cell's own genes, researchers introduce into cells short strands of RNA that correspond to the segments of genes being silenced.
The potential of RNAi to target specific genes was illustrated by a 2002 study, now considered a classic. Dutch researchers silenced mutant RAS genes in human cells leaving normal versions of the gene unaffected.
From a therapeutic perspective, the prospect of being able to turn off specific genes in tumor cells has long intrigued cancer researchers. But the science is still in its infancy, and RNAi can cause "off-target" effects. A major challenge is finding ways to get the RNA where it needs to go for a sufficient amount of time to benefit patients.
"The big issue with RNAi is delivery," says Dr. John J. Rossi of the Beckman Research Institute in Duarte, California. "We know we can get RNAi to work, but for the most part the delivery is not very specific and not very efficient."
Last year, biologists at Alnylam Pharmaceuticals in Germany addressed the delivery problem and showed that RNAi could be used to lower cholesterol levels in mice.
The team attached RNA molecules to cholesterol and injected them into the rodents' bloodstreams. The composite molecules were taken up by the liver, where blocking a gene helped lower blood cholesterol levels, according to findings in the Nov. 11, 2004, issue of Nature.
"The mouse study proved that delivery will be solvable for RNAi," says Dr. Judy Lieberman of Harvard Medical School in Boston, who co-authored an article in the March 16 Journal of the American Medical Association about using RNAi to treat disease. "They delivered the therapy to specific cells and saw a clinical effect."
Dr. Lieberman says that reliable cell-specific delivery would be "incredibly powerful" for cancer because researchers could target not only cancer genes but also other genes necessary for cell growth or cell division. "You might target four genes simultaneously and really restrict a cancer cell's ability to survive," she says.
While RNAi may not treat cancer for many years, if ever, the science is moving forward at a fast pace. Several groups are building large collections of RNA molecules that can be used to explore all manner of biological questions.
RNAi is increasingly being used, for instance, to investigate the mechanisms of action of drugs. Some medications work for unknown reasons, and RNAi can be used to identify the genes involved.
In fact, the technology can be used to identify the genes required for just about anything that happens in cells, according to Dr. Louis Staudt of NCI's CCR.
His team developed an RNAi-based method to identify the genes that cancer cells need to survive and proliferate. In this method, retroviruses are used to express about 2,000 different RNAi's in cancer cells, and then DNA microarrays are used to determine which of the RNAi's target essential genes regulate cancer cell growth and survival.
"It's an exciting time when you can look across thousands of genes simultaneously and probe their functions in human cancer," says Dr. Staudt.
High-throughput screening is another way to identify essential genes in cancer and potential drug targets. For example, the Translational Genomics Research Center (TGen) in Maryland has a large collection of RNA molecules
and exposes the molecules to cancer cells in parallel, thousands at a time.
"We are using RNAi to discover new points of vulnerability in cancer cells," says Dr. Spyro Mousses of TGen. Another project is to use RNAi to identify genes that, when silenced, make cancer cells more sensitive to chemotherapy.
Though not in the area of cancer, two clinical trials are testing RNAi as therapy for age-related macular degeneration. The delivery method is injection into the eye, and the findings will likely add to the growing knowledge about the fast-moving science of gene silencing.
By Edward R. Winstead
|
 |
 |
Studies Examine Racial Disparity in Survival Among Endometrial Cancer Patients
NCI researchers, in conjunction with Walter Reed Army Medical Center and other institutions, have completed two studies that suggest biological factors can contribute to the disparity in endometrial cancer survival rates between African Americans and Caucasians. Both studies were presented at the Society of Gynecologic Oncologists 2005 Annual Meeting on Women's Cancer, March 19-23, 2005, in Miami.
In the first study, the researchers compared the survival rates for 168 African American and 997 Caucasian women with advanced or recurrent endometrial cancer who were enrolled
in one of four trials performed by the NCI-funded Gynecologic Oncology Group. The analysis showed that, in a setting where all patients received equal care, African American women have a 25 percent greater chance of dying than Caucasian women with the same diagnosis.
In the second study, the investigators evaluated global gene expression for a small cohort that included 18 African American and 27 Caucasian women with endometrial cancer. They looked for any specific differences that may account for biological disparity. In the initial analysis, the researchers could not identify expression profiles unique to each race. However, when they excluded early-stage cancers and focused only on advanced-stage cancers, they observed expression profiles that did cluster according to race.
Although based on a small number of patients, author Dr. Larry Maxwell, LTC, of Walter Reed's Department of Obstetrics and Gynecology and NCI stated, "Both of these studies suggest that underlying molecular differences may partially explain the disparity in survival outcome for endometrial cancer for these two groups."
Medicare Adds Coverage of Smoking Cessation Services
The Centers for Medicare and Medicaid Services (CMS) announced March 23 that it is adding coverage for smoking and tobacco use cessation
counseling for certain Medicare beneficiaries to help them quit the habit.
The coverage decision, which was proposed for public comment in December, involves Medicare beneficiaries who have an illness caused or complicated by tobacco use, including heart disease, cerebrovascular disease, lung disease, weak bones, blood clots, and cataracts - diseases that account for the bulk of Medicare spending today. It also applies to beneficiaries who take certain medications, including insulin and medicines for high blood pressure, blood clots, and depression, the effectiveness of which is complicated by tobacco use.
"Covering smoking and tobacco use cessation counseling for seniors has great potential to save and improve lives for millions of seniors," said Dr. Mark B. McClellan, CMS administrator. "This is another step in turning Medicare into a prevention-oriented health program."
The Centers for Disease Control and Prevention has estimated that 9.3 percent of Americans aged 65 and older smoke cigarettes. About 440,000 people die annually from smoking-related disease, with 300,000 of those deaths in smokers aged 65 and older. Research has shown that
smoking cessation has significant health benefits, even after decades of smoking.
This announcement builds on a series of HHS initiatives designed to help Americans quit smoking, including the launch of a new national network of quitlines (1-800-QUITNOW) and designating all HHS campuses as tobacco-free. Medicare's upcoming prescription drug benefit will also cover smoking cessation treatments that are prescribed by a physician.
FDA Guidance Aimed at Expanding Individualized Medicine
The Food and Drug Administration (FDA) has issued a final guidance on the submission of genetic data associated with drugs and biologics under clinical investigation or being reviewed by the agency for marketing approval. The guidance document on the submission of such pharmacogenomic data, explained Dr. Janet Woodcock, acting deputy commissioner for operations at FDA, is aimed at speeding the movement toward personalized medicine.
Researchers increasingly are relying on sophisticated genetic tests to root out gene expression profiles that correlate with a drug's efficacy or toxicity. Increased collection of pharmacogenomic data, Dr. Woodcock said, "will allow medicines to be uniquely crafted to maximize their therapeutic benefits and minimize their potential risks for each patient."
Cancer is an area in which pharmacogenomics already is playing an expanded role. Tests are available to determine whether patients with metastatic breast cancer, for instance, overexpress the HER-2 gene, making them candidates for the targeted agent trastuzumab (Herceptin). The FDA also recently approved the first laboratory test to look for gene variations that are predictive of how well a patient will metabolize drugs for a broad range of conditions,
including cancer.
Pharmaceutical and biotechnology companies have been reluctant to collect and submit pharmacogenomic data to the FDA, the guidance explains, "because of uncertainties in how the data will be used by the FDA in the drug review process." The final guidance is intended to address industry concerns by clarifying how pharmacogenomic data will be evaluated, including what data will be required for regulatory decision making, as well as details on a new mechanism for industry to voluntarily submit pharmacogenomic research data that can help advance this relatively new area of research.
Researchers Find Genetic Link Between Blood Clotting Disorders and Cancer Onset
Researchers at the University of Turin Medical School have discovered that the MET oncogene can trigger both tumor development and blood clotting problems in mice. This finding, appearing in the March 17 Nature, provides the first genetic link to a 140-year-old enigma: the close relationship between the activation of blood coagulation and cancer.
Dr. Carla Boccaccio and colleagues engineered mice to overexpress the MET oncogene in adult liver cells. Three months after MET activation, the mice began displaying multiple, abnormally growing nodules in their livers, indicating the onset of tumors. However, even before the first nodules appeared, all of the engineered mice began developing numerous venous blood clots and internal hemorrhages. Most of the mice died after 6 months as a result of these blood disorders, while the few that survived developed hepatocarcinomas.
The researchers found that MET activation increased the activity of many blood clotting genes, including a significant increase in production of two key enzymes: PAI-1 and COX-2, both of which have been associated with cancer. Treating the mice with the COX-2 inhibitor rofecoxib both reduced the severity of the blood clotting and increased degeneration of the precancerous nodules.
Previous studies had demonstrated that MET activation could help drive tumor invasion and metastasis, and the authors suggest that MET's control over the blood clotting pathway is likely interdependent. "Oncogenic activation of MET triggers a genetic programme that not only transforms cells but also creates an extracellular environment that is fertile for tumor cell expansion and invasion," the authors wrote.
Enzyme Mutations Improve Aspirin's Protection Against
Colorectal Cancer
A genetic mutation that impairs the body's ability to metabolize aspirin appears to decrease the risk of colorectal cancer among women who regularly use aspirin, researchers from Harvard University and Massachusetts General Hospital report. The research team conducted a nested, case-control study of participants in the long-running Nurses' Health Study. They found that, among women with a common variant polymorphism in the UGT1A6 enzyme, which plays a key role in aspirin metabolism, regular aspirin use was associated with a 34 percent decreased risk of colorectal adenoma compared with nonregular users. Regular aspirin use among those with the normal, or wild-type, enzyme was not associated with reduced risk.
A number of studies have indicated that regular aspirin use can reduce the risk of colon polyps, established precursors to colorectal cancer. Two randomized, controlled clinical trials published in 2003 in the New England Journal of Medicine, for example, showed regular aspirin use reduced the development of colorectal polyps among those at high risk of colorectal cancer by as much as 35 percent.
In the new study, published in the March 16 Journal of the National Cancer Institute, the risk reduction associated with regular aspirin use in participants with UGT1A6 polymorphisms was greater at higher aspirin doses. As with lower doses, higher doses had no benefit for regular aspirin users with the wild-type enzyme.
"Certain subsets of the population, defined by genotype, may obtain differential benefit from aspirin chemoprevention," Dr. Andrew T. Chan and colleagues concluded. Whether UGT1A6 polymorphism status should influence who should receive aspirin for chemoprevention - and at what dose - remains uncertain, they added, and should be addressed in larger studies.
|
 |
 |

Monoclonal Antibody Therapy for Treatment-Resistant
Blood Cancers
Name of the Trial
Phase I Study of Siplizumab (MEDI-507) in Patients with CD2-Positive Lymphoproliferative Disorders (NCI-04-C-0031). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-04-C-0031.
Principal Investigator
Dr. John Edward Janik, NCI's CCR
Why Is This Trial Important?
Lymphoproliferative disorders, such as leukemias and lymphomas, are diseases in which cells of the lymphatic system (lymphocytes) grow excessively. These cancers can be especially hard to treat effectively if they arise from a malfunction in one type of lymphocyte called a T cell.
Siplizumab is a monoclonal antibody that binds to a protein called CD2, which is found abundantly on certain types of lymphocytes including T cells and Natural Killer (NK) cells. Initially developed to treat psoriasis, siplizumab has been shown in clinical studies to trigger T-cell death. This phase I dose-escalation study is investigating safety and tolerability, and will determine the maximum dose of siplizumab that can be given to patients with CD2-positive lymphoproliferative disease.
 "Preclinical studies of siplizumab at NCI produced very promising results, with a short course of treatment yielding a 50 percent cure rate in mice with T-cell non-Hodgkin's lymphoma," said Dr. Janik. "Longer courses of treatment led to the treated mice living out their natural life spans. "Preclinical studies of siplizumab at NCI produced very promising results, with a short course of treatment yielding a 50 percent cure rate in mice with T-cell non-Hodgkin's lymphoma," said Dr. Janik. "Longer courses of treatment led to the treated mice living out their natural life spans.
"Early results from this trial have produced promising responses in some patients, and we hope to follow this research with additional studies combining siplizumab and chemotherapy," Dr. Janik added.
Who Can Join This Trial?
Researchers seek to enroll up to 24 additional patients aged 18 or older with CD2-positive lymphomas or leukemias that have been unresponsive to previous treatment. See the full list of eligibility criteria at http://cancer.gov/clinicaltrials/NCI-04-C-0031.
Where Is This Trial Taking Place?
This trial is being conducted at the NIH Clinical Center in Bethesda, Md.
Contact Information
For more information, call the NCI Clinical Studies Support Center (CSSC) at 1-888-NCI-1937. The call is toll free and completely confidential.
An archive of "Featured Clinical Trial" columns is available at
http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |

Cancer Pioneer Discusses Nutrition
Speaking last week on the NIH campus, Dr. Paul Talalay, from the Department of Pharmacology and Molecular Sciences at Johns Hopkins School of Medicine, detailed some of the latest data and support for the role of diet in cancer prevention. The speech was part of the Stars in Nutrition & Cancer seminar series sponsored by the NCI Division of Cancer Prevention.
Carcinogenesis, Dr. Talalay argued, is a normal, multistage process - but a silent one. Until a clinical diagnosis is made, we don't know which of our thousands of normal cells have begun to undergo genetic and epigenetic changes, on the road to neoplasm and eventually malignant tumor. But, he explained, we now know that compounds found in everyday foods have been shown to alter carcinogenesis. Researchers in Dr. Talalay's laboratory, for example, have demonstrated that the compound sulforaphane, found abundantly in broccoli sprouts, "is a very powerful mechanism for reducing the risk of cancer, and probably many other chronic degenerative conditions as well," he said. The compound works, he continued, by activating so-called Phase 2 genes that code for proteins that protect cells against some of the most damaging toxic and chemical effects that lead to neoplasms. Similar to drugs, bioactive food components have specific sites of action. This could one day help identify those people who would benefit most from dietary change.
 NCI Featured in New PBS Documentary
NCI Featured in New PBS Documentary
Five NCI scientists are featured in an upcoming PBS documentary, CancerStory, slated to air on 82 PBS stations starting this April. The documentary, which was produced by the Norris Cotton Cancer Center of the Dartmouth-Hitchcock Medical Center, is designed to put the complexities of cancer into terms the average person can understand and use. The program consists of four hour-long segments that focus on the biological mechanisms of cancer, the experiences of cancer survivors, promising new cancer treatments, and screening and prevention. NCI Director Dr. Andrew C. von Eschenbach and NCI researchers Drs. Peter Greenwald, C. Norman Coleman, Julia Rowland, and Meg Mooney all contributed to the documentary. Their interviews and a comprehensive list of stations that will be airing the documentary can be found at http://www.cancerstory.org. For specific airtimes, check your local PBS listings.
CCR Fellow Wins Ruth and William Silen, M.D., Award
Dr. Samuel T. Waters, one of four CCR fellows who participated in last month's New England Science Symposium in Boston, has won the Ruth and William Silen, M.D., award for his talk entitled, "Genetic Analysis of Mouse Gbχ Transcription Factors During Neural Development." Dr. Waters is a postdoctoral fellow in the lab of Dr. Mark Lewandoski at NCI-Frederick.
NCI Holds Science Writers' Seminar at Memorial Sloan-Kettering Cancer Center
On March 23 NCI held its 13th science writers' seminar. Hosted jointly with Memorial Sloan-Kettering Cancer Center (MSKCC), the seminar was entitled "New Frontiers in Prostate Cancer Treatment and Prevention," and included presentations by a panel of experts from NCI and MSKCC. Panelists included Drs. Peter Scardino, Michael Zelefsky, and Howard Scher, all of MSKCC, who discussed new advances in the diagnosis and treatment of prostate cancer; and Drs. Howard Parnes and Leslie Ford of NCI, who spoke about the recently completed Prostate Cancer Prevention Trial and new avenues in prostate cancer prevention.
The next science writers' seminar on children and cancer is scheduled for April 26 at the Children's Inn on the NIH campus in Bethesda, Md. Journalists who wish to attend can contact the NCI Press Office at (301) 496-6641 or ncipressofficers@mail.nih.gov.
|
 |
 |

Oncology Nursing and 2015
 The current shortage of nurses and nursing faculty has reached crisis proportions. According to the U.S. Bureau of Labor Statistics, this country will be short 1.1 million nurses by 2012. The aging of the current nursing workforce, alternative job opportunities available for registered nurses, and a relatively flat earning curve over the past 20 years exacerbate the problem. Interestingly, many individuals looking toward second or third careers are turning to nursing. But the lack of nursing faculty to educate an increased workforce is evidenced by the 32,000 qualified applicants turned away from baccalaureate and graduate nursing programs in 2004 - resulting in the loss of an estimated 3,000 Ph.D.-prepared nurses. When community colleges are included in the statistics, the number turned away skyrockets to 125,000 applicants. The aging of the population, the correlation between age and cancer risk, and an increased per capita demand for health care create a "perfect storm" in the cancer care community.
The current shortage of nurses and nursing faculty has reached crisis proportions. According to the U.S. Bureau of Labor Statistics, this country will be short 1.1 million nurses by 2012. The aging of the current nursing workforce, alternative job opportunities available for registered nurses, and a relatively flat earning curve over the past 20 years exacerbate the problem. Interestingly, many individuals looking toward second or third careers are turning to nursing. But the lack of nursing faculty to educate an increased workforce is evidenced by the 32,000 qualified applicants turned away from baccalaureate and graduate nursing programs in 2004 - resulting in the loss of an estimated 3,000 Ph.D.-prepared nurses. When community colleges are included in the statistics, the number turned away skyrockets to 125,000 applicants. The aging of the population, the correlation between age and cancer risk, and an increased per capita demand for health care create a "perfect storm" in the cancer care community.
 |
 |
Featured Meetings and Events |
 |
|
A comprehensive calendar of cancer-related scientific meetings and events sponsored by NCI and other scientific organizations, is available at: http://calendar.cancer.gov/
|
|
As president of the 32,000-member Oncology Nursing Society (ONS), I applaud the efforts of the nursing and cancer care communities to resolve this problem. We don't have exact numbers for the oncology nursing shortage, but we cannot ignore the consequences of a shortage for oncology patients across the continuum of care - beginning with prevention and early detection and extending through treatment and on to survivorship. The multidisciplinary approach that provides high-quality cancer care would be almost impossible without nursing clinicians, educators, administrators, and scientists.
In a recent meeting, Dr. Andrew von Eschenbach and I discussed the goal of eliminating suffering and death due to cancer by 2015 and how ONS and NCI might partner to realize that goal. This goal cannot be achieved without the efforts of oncology nurses. Our knowledge, skills, and commitment are integral to the clinical and research arenas of oncology. Our historical knowledge of cancer biology has been integrated into our understanding of cancer at the molecular level, and that knowledge drives patient and family education, administration of therapy, and management of sequelae to those therapies. Oncology nurses have translated a significant body of nursing research in symptom and side effect management, psychosocial and behavioral issues, and health promotion into practice settings. Our goals are to improve the
outcomes, quality, effectiveness, and overall costs of care.
Oncology nursing's contribution to the existential issues of care must not be lost in the discussion of clinical and research issues. Oncology nurses are existential activists: They establish emotional connections with patients and families, affirm and value the person amid the realities of illness, listen quietly as patients describe their fears and vulnerabilities, give permission to speak the unspeakable, and remain in the moment - no matter how difficult. Nursing presence can provide meaning and comfort, diminish anxiety and loneliness, and offer reassurance. While the paradigm of oncology therapy will change over the next decade, the value of and the need for highly qualified, well-educated, and compassionate oncology nurses will not.
Karen J. Stanley
President, Oncology Nursing Society
|
 |
|