 |

Adoptive Cell Transfer Shows Dramatic Results in Refractory Melanoma

A study in the April 2005 Journal of Clinical Oncology, from the lab of Dr. Steven Rosenberg, chief of National Cancer Institute's (NCI) Surgery Branch, reports promising results of adoptive cell transfer (ACT) immunotherapy among 35 patients with metastatic melanoma. ACT is an innovative biological therapy developed by NCI researchers which has been shown to shrink solid tumors that have resisted other treatments. Of that cohort, 3 experienced a complete response and 15 had a partial response lasting from 2 months to 2 years - a response rate of 51 percent. Thirteen of the 18 who responded had cancers resistant to chemotherapy and 34 of the 35 patients had been resistant to high-dose interleukin-2 (IL-2) therapy. The study provides clinical confirmation of Dr. Rosenberg's previous cancer immunotherapy study published in Science in 2002. That study showed an effect from treatment on tumors in 6 out of 13 patients - a response rate of 43 percent.
These and other studies are laying the foundation for biological therapies that use the body's immune system, directly or indirectly, to fight cancer or to lessen the side effects of other treatments. Dr. Rosenberg says the field was jump-started 20 years ago by the discovery that IL-2 cytokines - which can also be made in the lab - could shrink established, metastatic solid tumors. Since then, researchers have identified dozens of antigens on the surface of certain cancer cells that the body's immune system recognizes and attacks as intruders. Immunotherapeutic vaccines are being studied that would use these features to target exclusively the cancer cells for elimination. "Although cancer vaccines have yet to prove effective," Dr. Rosenberg said, "much work on that front is ongoing."
Read more 


The Global Impact of 2015

During a recent trip to Italy, I visited a university in the Calabria region, the Magna Graecia University in the city of Catanzaro. Italy, of course, is known for its rich history, beautiful architecture, and spectacular landscapes. But I came away from my visit even more impressed by this university's keen commitment to biomedical research and advanced technologies and their enthusiasm for the NCI goal to eliminate the suffering and death due to cancer.
Magna Graecia was only officially established as an independent university in 1998. But under the guidance of respected scientists and leaders, it has quickly become a leading research institution, including a major commitment to interdisciplinary research and technology development, particularly in the areas of proteomics and nanotechnology. Its president, Dr. Salvatore Venuta, is a highly respected cancer researcher who was educated and trained in Italy and at top-flight U.S. academic institutions and cancer centers.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Adoptive Cell Transfer Shows Dramatic Results in Refractory Melanoma
A study in the April 2005 Journal of Clinical Oncology, from the lab of Dr. Steven Rosenberg, chief of National Cancer Institute's (NCI) Surgery Branch, reports promising results of adoptive cell transfer (ACT) immunotherapy among 35 patients with metastatic melanoma. ACT is an innovative biological therapy developed by NCI researchers which has been shown to shrink solid tumors that have resisted other treatments. Of that cohort, 3 experienced a complete response and 15 had a partial response lasting from 2 months to 2 years - a response rate of 51 percent. Thirteen of the 18 who responded had cancers resistant to chemotherapy and 34 of the 35 patients had been resistant to high-dose interleukin-2 (IL-2) therapy. The study provides clinical confirmation of Dr. Rosenberg's previous cancer immunotherapy study published in Science in 2002. That study showed an effect from treatment on tumors in 6 out of 13 patients - a response rate of 43 percent.
 These and other studies are laying the foundation for biological therapies that use the body's immune system, directly or indirectly, to fight cancer or to lessen the side effects of other treatments. Dr. Rosenberg says the field was jump-started 20 years ago by the discovery that IL-2 cytokines - which can also be made in the lab - could shrink established, metastatic solid tumors. Since then, researchers have identified dozens of antigens on the surface of certain cancer cells that the body's immune system recognizes and attacks as intruders. Immunotherapeutic vaccines are being studied that would use these features to target exclusively the cancer cells for elimination. "Although cancer vaccines have yet to prove effective," Dr. Rosenberg said, "much work on that front is ongoing."
These and other studies are laying the foundation for biological therapies that use the body's immune system, directly or indirectly, to fight cancer or to lessen the side effects of other treatments. Dr. Rosenberg says the field was jump-started 20 years ago by the discovery that IL-2 cytokines - which can also be made in the lab - could shrink established, metastatic solid tumors. Since then, researchers have identified dozens of antigens on the surface of certain cancer cells that the body's immune system recognizes and attacks as intruders. Immunotherapeutic vaccines are being studied that would use these features to target exclusively the cancer cells for elimination. "Although cancer vaccines have yet to prove effective," Dr. Rosenberg said, "much work on that front is ongoing."
The ACT trials illustrate many of the principles of biological immunotherapy. Dr. Rosenberg's team first identified the specific T cells that each patient's immune system generated in response to his or her cancer. The researchers removed a sample of these cells from the tumor site and put them through an ex vivo enhancement process to create tumor-infiltrating lymphocytes (TILs). "These cells exist in the body and can specifically recognize the tumor; most are ineffective in their native state," explains Dr. Rosenberg. His team selected the most aggressive T cells from among those that had infiltrated the tumors, testing them ex vivo against tumor samples from that patient. The most potent cells were then collected and multiplied.
An important refinement on the technique was to prepare the patient's immune system to accept the engineered TIL cells. The researchers accomplished this by lymphodepletion, a chemotherapy regimen that eliminates many of the patient's resident ineffective T cells that failed to combat the cancer. Once the new multiplied TIL cells are returned to the patient's body and to the immature T cells in the biological environment of the patient's immune system, they are supplemented by high doses of IL-2 cytokines, proven immune system stimulators.
"We've learned a lot about managing such treatments and dealing with the toxicities safely," notes Dr. Rosenberg. "Treatment-related mortality in IL-2 patients is now less than 0.5 percent," and there were no treatment-related deaths in the new trial. "We saw the expected toxicities associated with lymphodepleting chemotherapy," the researchers wrote in the article, but "these were mostly transient and readily managed with standard supportive techniques."
A new era of cancer therapy is nigh. Although Dr. Rosenberg cautions against overselling early experimental results, he maintains the hope that - as his team and others continue to evolve biological approaches for their specific cancer models - "principles will crystallize and guidelines will emerge to lead us to more general immunotherapies for other types of cancer."
By Addison Greenwood
|
 |
 |

The Global Impact of 2015
During a recent trip to Italy, I visited a university in the Calabria region, the Magna Graecia University in the city of Catanzaro. Italy, of course, is known for its rich history, beautiful architecture, and spectacular landscapes. But I came away from my visit even more impressed by this university's keen commitment to biomedical research and advanced technologies and their enthusiasm for the NCI goal to eliminate the suffering and death due to cancer.
Magna Graecia was only officially established as an independent university in 1998. But under the guidance of respected scientists and leaders, it has quickly become a leading research institution, including a major commitment to interdisciplinary research and technology development, particularly in the areas of proteomics and nanotechnology. Its president, Dr. Salvatore Venuta, is a highly respected cancer researcher who was educated and trained in Italy and at top-flight U.S. academic institutions and cancer centers.
Dr. Venuta's and other Magna Graecia leaders' dedication to using advanced technologies to take cancer research to the next frontier was particularly impressive, and was matched by the building of state-of-the-art clinical and research facilities. What may sometimes get lost in the debate about our progress against cancer is that our leadership toward achieving the 2015 goal is having a global impact. Because, although cancer is a major killer in the United States and most developed countries, it's in developing countries where this disease is projected to increase most rapidly in the coming decades. So our research advances will touch people around the world.
My visit also reaffirmed for me that science is a global enterprise. At a grand rounds presentation on the National Institutes of Health (NIH) campus last week, for example, Dr. John Potter from Fred Hutchinson Cancer Research Center spoke about his proposal to conduct a study he calls the "last cohort." This international cohort study would follow at least 1 million people between 50 and 75 years of age to definitively identify genetic and environmental cancer risk factors. International representation, he advised, is critical to any large cohort study of this nature because of the importance of having a heterogeneous study population. Indeed, if we are to achieve our 2015 goal, it is imperative that we have a global understanding of the genetic variants and environmental exposures related to cancer.
As was highlighted in the Cancer Bulletin last November, NCI is already significantly engaged on the international front through efforts such as visiting scientists' programs and large, international clinical trials and consortia. As a report released last week in the European Union (EU) showed, funding for cancer research in the United States as a percentage of the gross domestic product far exceeds cancer research spending in EU countries. As a result, the United States continues to be
regarded as the leader in cancer research, and I believe we have made - and will continue to make - the strategic decisions needed to strengthen that leadership.
I am confident that we can transform cancer into a disease that can be both prevented and managed like other chronic illnesses. To paraphrase how an Italian reporter who interviewed me summed it up in his article: Even for those who become "sick" with cancer, their life will still be long.
Dr. Andrew C. von Eschenbach
Director, National Cancer Institute
|
 |
 |

Novel Aspirin Offers Promise for Colorectal Cancer Prevention
Most people probably would not associate car exhaust fumes with cancer prevention. Those fumes, however, contain nitric oxide (NO), gas molecules also produced by human cells that are essential to the regulation of a host of important biological functions, from the immune response to blood pressure.
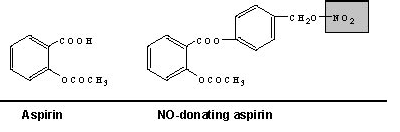 A great deal of research these days is focused on taking advantage of some of this air pollutant's remarkable regulatory talents. Human clinical trials are now testing, for example, "NO-donating" compounds to treat diseases and conditions as diverse as asthma and Alzheimer's. Last week the first human clinical trial was initiated testing an NO-donating aspirin as a chemopreventive agent against colorectal cancer.
A great deal of research these days is focused on taking advantage of some of this air pollutant's remarkable regulatory talents. Human clinical trials are now testing, for example, "NO-donating" compounds to treat diseases and conditions as diverse as asthma and Alzheimer's. Last week the first human clinical trial was initiated testing an NO-donating aspirin as a chemopreventive agent against colorectal cancer.
The research into this compound, a derivative of aspirin-releasing nitric oxide dubbed NCX4016, builds on data from epidemiologic studies and clinical trials showing that regular use of traditional aspirin can significantly reduce colon polyp formation in those at high risk of developing them, including those already treated for colorectal cancer. According to Dr. Basil Rigas, chief of the Division of Cancer Prevention at the State University of New York at Stony Brook, in laboratory and animal model studies he has led, NCX4016 has proven hundreds of times more potent than traditional aspirin in inhibiting growth of colon cancer cells in cell cultures. And in a mouse model of colon cancer, mice given NCX4016 daily for 3 weeks had a 59 percent tumor reduction on average. In a similar study using rats, tumor growth was reduced by 75 percent, and new tumors failed to grow. In both cases, the drug was effectively free from toxicity.
The gastrointestinal (GI) toxicity often seen with regular aspirin use and other nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen fueled the development of NO-donating NSAIDs in the mid-1990s. "NO," says Dr. James Crowell, a program director in the NCI Division of Cancer Prevention, "stimulates vasodilation and mucous secretion by the cells that line the GI tract." So an NSAID that releases NO may effectively nullify the NSAID's ability to cause serious, sometimes life-threatening problems such as bleeding ulcers.
What initially brought NO to the research forefront, however, was something altogether different: the discovery in the late 1980s of its role as a signaling molecule in the cardiovascular system - the first time a gas molecule was found to participate in the communication network within cells that regulate functions such as growth, division, and death. The discovery, for which a trio of scientists was awarded the 1998 Nobel Prize, spurred researchers from across the globe to see if NO played a similar role in other organ systems.
What they found is that "nitric oxide regulates nearly every tissue in your body," says Dr. David Wink, a principal investigator in NCI's Center for Cancer Research Radiation Biology Branch who has been studying the ubiquitous molecule for nearly 15 years. His lab's work has led to some intriguing discoveries. "We have found that changes in the doses of our NO donor compounds by very small amounts cause profound changes in tumor cells and signal transduction," Dr. Wink says.
Following on their studies showing that, in cell culture, tumors can use NO to promote angiogenesis, Dr. Wink's laboratory is now in the early stages of investigating how inhibiting NO affects standard cancer therapy. "We've found that if we inhibit NO after radiation or chemotherapy treatments, we see tremendous increases in the treatments' efficacy," he says.
As for chemoprevention, NCX4016's promise, Dr. Rigas notes, is not limited to colorectal cancer. In an animal model system of pancreatic cancer, treatment or pre-treatment with NCX4016 prevented 90 percent of pancreatic cancers. His laboratory has received several grants to study NO's mechanism of action, work primarily focused on elucidating what intracellular signaling pathways it affects. So far, several pathways have stood out, including NF-κB and Wnt, both of which are thought to be involved in carcinogenesis.
The molecular biology of NO in cancer is still not well understood, stresses Dr. Crowell. Additional research will provide insight into the potential long-term impact of NCX4016's use and
help guide its potential use in combination with other therapies.
The trial initiated last week - supported by NCI and conducted in conjunction with NicOx, the French company that is developing a number of NO-donating agents for a wide variety of indications - will include a pharmacokinetic component aimed at answering some of those questions. It will recruit 240 patients at high risk of colorectal cancer and test whether, after 6 months of treatment, the drug can prevent or arrest the growth of microscopic lesions found in the colon lining, called aberrant crypt foci, which are thought to be polyp precursors.
Although the potency NCX4016 has displayed in laboratory and animal model studies is enticing, says Dr. Rigas, in the relatively new area of chemoprevention, establishing safety is paramount. "With chemoprevention, you're administering an agent to an otherwise healthy individual at risk for developing a cancer," he says. "And that person is committed to receiving that agent for a very long time."
By Carmen Phillips
|
 |
 |
Kidney Cancer Drug Delays Disease Progression
The experimental drug BAY 43-9006 (sorafenib) was shown to delay the progression of advanced kidney cancer according to preliminary results from a phase III clinical study. The findings were announced March 21 by the drug's two sponsors, Bayer Pharmaceuticals and Onyx Pharmaceuticals. An independent data monitoring committee for the trial concluded that the study had met its surrogate endpoint resulting in statistically significant, longer progression-free survival in patients taking sorafenib compared with patients taking a placebo.
As a result, the two companies announced plans to file a New Drug Application with the FDA, seeking accelerated approval for sorafenib. They also will pursue completion of the phase III trial toward the primary endpoint of demonstrating whether the drug improves overall survival rates for kidney cancer patients. In addition, the companies plan to submit data from the phase III study at the American Society of Clinical Oncology annual meeting in May.
The multinational trial enrolled over 800 patients with advanced kidney cancer. Half of the patients in the randomized, double-blind study received sorafenib while the other half received placebo.
Sorafenib is a targeted oral therapy with a two-fold method of action, inhibiting both the RAF kinase signaling pathway while also exerting an antiangiogenic effect through inhibition of VEGFR-2 and PDGFR-ß kinases. "BAY 43-9006 has demonstrated both antiproliferative and antiangiogenic properties - two important anticancer activities," reports Onyx. The drug is reported to have moderate-to-low side effects in most patients. The companies also announced plans to test sorafenib for treatment of melanoma.
Collagen VII Mutation Linked to Skin Cancer's Spread
In an experimental model of human squamous cell skin cancer, collagen VII, a protein that helps bind the superficial epidermis to the underlying dermis, has now been implicated in the eventual invasion of squamous cancer cells to other parts of the body. This finding is published in the March 18 Science.
The research team from Stanford University School of Medicine worked with skin keratinocytes and skin cancers from patients with the genetic disease recessive dystrophic epidermolysis bullosa (RDEB), in whom the collagen VII gene is mutated, causing their skin to be fragile and blister. More than 55 percent of RDEB patients die from metastatic squamous cell carcinoma (SCC) by the age of 40, while a subset never develops it.
Examining this disparity, the researchers collected SCC samples from 10 RDEB patients and found that they all had detectable collagen VII expression. However, in 10 RDEB patients who had not developed SCC, 4 skin biopsy samples lacked collagen VII expression. After transducing keratinocytes from RDEB patients with oncogenic retroviruses and injecting them into mice, those keratinocytes from patients lacking collagen VII did not form tumors, but those from patients expressing a collagen VII protein, albeit truncated, formed invasive SCC.
The authors showed that physical interactions between collagen VII and laminin-5, an extracellular basement membrane protein, facilitates SCC invasion, and further demonstrated that an N-terminal fragment of collagen VII, FNC1, was sufficient for tumor formation and invasion. The authors propose that collagen VII or FNC1 may be a novel therapeutic target against tumor invasion.
In a related editorial, however, Dr. Stuart H. Yuspa of NCI and Dr. Ervin H. Epstein, Jr., of the University of California, San Francisco, warn that such a therapy would need to selectively block tumor invasion without weakening the anchor between epidermis and dermis. "We are faced with a possible Pyrrhic victory," they write, "perhaps winning the battle against SCC but losing the battle against the disfiguring skin defects of [RDEB]."
Immunosuppressive Drug Sirolimus Can Inhibit Kaposi's Sarcoma in Kidney Transplant Patients
Researchers at the University of Foggia in Italy have shown that the drug sirolimus (rapamycin) can inhibit Kaposi's sarcoma in kidney transplant recipients while still preventing host-organ rejection. These results, appearing in the March 31 New England Journal of Medicine, suggest sirolimus' dual antitumor and antirejection abilities will make it an effective therapy for transplant patients at risk for developing tumors.
Kaposi's sarcoma is a malignancy associated with uncontrolled growth of blood vessels and frequently results in purple lesions appearing on the skin. The incidence of Kaposi's sarcoma is much higher among organ transplant recipients and AIDS patients, indicating that suppression of the immune system may promote development of this disease. Treating Kaposi's sarcoma in transplant patients is complicated because immunosuppression needs to be maintained in order to prevent host rejection of the transplanted organ.
The immunosuppressive drug sirolimus has been shown to have some antitumor properties in animal and cell-line studies. The researchers, led by Dr. Giuseppe Grandaliano, examined the clinical effect of sirolimus in 15 kidney transplant patients who developed Kaposi's sarcoma. Upon sarcoma diagnosis, the patients stopped receiving cyclosporine and began sirolimus treatment. One month after sirolimus therapy began, the sarcoma lesions began regressing in 12 of the 15 patients, and 3 months after initiation all 15 patients achieved clinical and histological remission. Importantly, the switch in treatment did not provoke any acute episodes of rejection and graft function remained stable during sirolimus treatment.
Rapamycin Agent Improves Response to Low-Dose Cisplatin
The rapamycin-derivative RAD001 (everolimus) dramatically enhanced solid tumor responses to low doses of the DNA-damaging agent cisplatin in a study reported in the March 25 issue of Cell.
Researchers from Novartis and the Friedrich Miescher Institute in Basel, Switzerland, combined RAD001 with cisplatin against cell lines of A549 non-small-cell lung cancer. The most dramatic effects for inducing apoptosis (cell death) in the tumor were found at normally suboptimal doses of cisplatin "with a more than 10-fold difference observed at a concentration where cisplatin alone had no detectable effect," the scientists report. RAD001's additive effect disappeared at higher doses of cisplatin, they add.
Cisplatin and other DNA-damaging agents "have revolutionized chemotherapy against solid tumors" but have been limited in their use by a "narrow therapeutic window combined with severe side effects," the researchers note. Derivatives of the fungicide rapamycin have shown promise by sensitizing tumor cells to respond to lower doses of such chemotherapeutic agents.
"The sensitizing effects of RAD001 on loss of cell viability are presumed to be through inactivation of the mTOR pathway," the scientists comment. To explore that theory, they tested RAD001 on mTOR cell lines. "These findings suggest that RAD001 sensitizes tumor cells to DNA-damaging agents by blocking the upregulation of p21 through inhibition of mTOR," they conclude.
The researchers comment that "targeting mTOR with agents like RAD001 may offer the opportunity to treat p53 wild-type tumors with much lower doses of DNA-damaging agents, thereby reducing side effects while maintaining antitumor efficacy."
Colorectal Screening for African Americans Should Start at Age 45, Panel Urges
A panel of experts from the American College of Gastroenterology (ACG) has recommended that African Americans begin screening for colorectal cancer at age 45 rather than age 50. This was included along with other recommendations aimed at reducing the death and suffering caused by colorectal cancer among African Americans in a special report in the March 2005 American Journal of Gastroenterology.
The report was based on an "extensive review" of the literature on colorectal cancer screening and issues related to health screening in African Americans. This group has the highest incidence of colorectal cancer of any racial or ethnic group in the United States. At the time of diagnosis, African Americans tend to be younger and have more advanced disease as compared with whites. Survival rates in African Americans with colorectal cancer are also lower than in whites.
The proposal to begin screening this population group at age 45 is "very appropriate and fully justified given the high incidence and early age of onset of colorectal cancer in African Americans," comments Dr. Douglas Rex of Indiana University and a past ACG president. "This is just the beginning. As the document states, special efforts are needed to ensure that screening indeed takes place in this high-risk population."
Among other recommendations, the ACG panel endorsed the use of colonoscopy as a first-line screening procedure and urged professional gastroenterological societies to create educational programs to make clinical gastroenterologists aware of facts about colorectal cancer in African Americans.
|
 |
 |

The following is a newly released NCI research funding opportunity:
Tools for Zebrafish Research
PAR-05-080
Letter of Intent Receipt Dates: Aug. 19, 2005, Aug. 19, 2006, Aug. 19, 2007
Application Receipt Dates: Sept. 19, 2005, Sept. 19, 2006, Sept. 19, 2007
This is a reissue of PAR-02-142, which was issued because it was clear that there was a critical need for non-hypothesis driven, tool development proposals to be reviewed as a group, within a single framework. It focused on identifying additional mutants and developing new genetic tools in zebrafish. The objective is to continue to broaden the range, power, and utility of tools for biomedical and behavioral research using zebrafish, and to develop genetic and genomic resources of high priority to the zebrafish community. NCI's interest in this initiative is the study of zebrafish models to identify and place genes in functional pathways that affect growth and development.
This funding opportunity will use the NIH Individual Research Project Grant (R01) award mechanism(s).
For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2660.
Inquiries: Dr. Lorette Javois - lj89j@nih.gov.
The NIH Roadmap for Medical Research Funding provides a framework of the priorities NIH must address to optimize its research portfolio. It identifies the most compelling opportunities in three main areas: new pathways to discovery, research teams of the future, and re-engineering the clinical research enterprise. Newly released Roadmap funding opportunities are listed below. For information on additional Roadmap funding opportunities, go to http://nihroadmap.nih.gov.
|
 |
 |

Oblimersen Treatment for Older Patients with AML
Name of the Trial
Phase III Randomized Study of Daunorubicin and Cytarabine with or without Oblimersen in Older Patients with Previously Untreated Acute Myeloid Leukemia (CALGB-10201). See the protocol abstract at http://cancer.gov/clinicaltrials/CALGB-10201.
 Principal Investigator
Principal Investigator
Dr. Guido Marcucci, Cancer and Leukemia Group B
Why Is This Trial Important?
Acute myeloid leukemia (AML) is the most common type of blood and bone marrow cancer in U.S. adults. Treatment of newly diagnosed AML usually involves chemotherapy to induce a remission of the cancer (induction therapy), followed by more chemotherapy to keep the cancer in remission and prevent a relapse (consolidation or post-remission therapy).
In this trial, researchers are trying to determine whether adding the drug oblimersen (Genasense) to chemotherapy will improve survival in patients aged 60 and older who have previously untreated AML. Oblimersen blocks production of a protein called Bcl-2, which helps cancer cells survive. Bcl-2 is overexpressed in many types of tumors and contributes to cancer cell resistance to chemotherapy. By blocking production of Bcl-2, oblimersen may make cancer cells more susceptible to the cytotoxic effects of chemotherapy.
"Although AML long-term remission rates in younger patients are better, the long-term remission rates for AML in patients over 60 years old are very low, about 10-15 percent," said Dr. Marcucci. "AML is prevalent in the geriatric population in America, and as that population continues to grow, we can expect to see an increase in the number of older Americans with AML.
"That is why we need to come up with a new approach for treating AML in older patients," Dr. Marcucci added.
Who Can Join This Trial?
Researchers seek to enroll 500 patients aged 60 and older with a confirmed diagnosis of AML who have not yet received treatment for their disease. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/CALGB-10201.
Where Is This Trial Taking Place?
Multiple study sites in the United States are recruiting patients for this trial. See the list of study sites at http://cancer.gov/clinicaltrials/CALGB-10201.
Contact Information
See the list of study contacts at http://cancer.gov/clinicaltrials/CALGB-10201 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The call is toll free and completely confidential.
An archive of "Featured Clinical Trial" columns is available at
http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |

 |
 |
CCR Grand Rounds |
 |
|
April 12: Dr. Bruce E. Johnson, Director, Lowe Center for Thoracic Oncology Dana-Farber Cancer Institute, Associate Professor of Medicine, Harvard Medical School. "Impact of EGFR Mutations on the Treatment of Lung Cancer"
April 19: No lecture. American Association of Cancer Research (AACR) meeting April 16-20, Anaheim, Calif.
April 26: Dr. John T. Schiller, Senior Investigator, Laboratory of Cellular Oncology, Center for Cancer Research, NCI. "Recent Advances in Prophylactic HPV/Cervical Cancer Vaccines"
|
|
Fourth International Conference on Cervical Cancer
The University of Texas M. D. Anderson Cancer Center will sponsor and host the Fourth International Conference on Cervical Cancer, May 19-22, 2005, in Houston, Texas. The conference will be co-sponsored by the Johns Hopkins University School of Medicine, NCI, the Centers for Disease Control and Prevention, the European Institute of Oncology, Medical School of the University of Turin, and the Mirano Medical Center (Venice, Italy). The conference goal is to provide an update on research in cervical cancer by assembling leaders from multiple disciplines involved with all aspects of cervical cancer causation, prevention, and screening. The conference
will focus on optical techniques for screening and detection, novel surgical approaches and chemo-radiation, prophylactic and therapeutic HPV vaccines, behavioral interventions, and quality-of-life issues. For more information
go to http://www.mdanderson.org.
CTWG Seeks Feedback on Draft Recommendations
NCI's Clinical Trials Working Group (CTWG) is requesting feedback on its draft recommendations to improve the national clinical trials system. Developed over the last few months, the draft recommendations evaluate the strengths and weaknesses of the current system and address a wide range of issues. They are posted on the CTWG Web site
(http://integratedtrials.nci.nih.gov). All stakeholders in the clinical trials process are encouraged to submit feedback to ctwgfeedback@mail.nih.gov by April 15. Comments will be considered during
the preparation of the final recommendations, which will be presented to the National Cancer Advisory Board at its June 7 meeting.
caBIG Annual Meeting will be Webcast Live
The plenary sessions of the 2005 caBIG Annual Meeting on April 12-13 will be webcast live. Due to a large response, registration to attend the meeting has already been closed. The webcast will enable additional interested individuals to learn about the latest developments in NCI's caBIG initiative (NCI Cancer Bulletin, March 22, 2005). Webcast information and a meeting overview are available at http://caBIG.nci.nih.gov/2005_Annual_Meeting.
Science Writers' Seminar on Childhood Cancers
Extraordinary progress has been made in treating childhood cancers. A child diagnosed with cancer in the 1970s had less than a 50 percent chance of surviving for 5 years; today, 5-year survival rates for all childhood cancers are approaching 80 percent. NCI is sponsoring a Science Writers' Seminar to highlight the latest scientific discoveries
in children's cancer and treatment on April 26. Speakers will include Drs. Lee Helman and Alan Wayne of NCI; Dr. Donald Small of Johns Hopkins University; and Kathy Russell, acting executive director of the Children's Inn at NIH. An NCI patient will also speak on the experience of having cancer as a child. The seminar will take place from 11 a.m. to 1:30 p.m. in the multipurpose room of the Children's Inn on the NIH campus in Bethesda, Md. Journalists wishing to register for the press briefing should contact Dorie Hightower or Ann Benner in the NCI Press Office at (301) 496-6641 or at ncipressofficers@mail.nih.gov.
|
 |
 |

"Toward the Last Cohort"
 |
 |
Featured Meetings and Events |
 |
|
A comprehensive calendar of cancer-related scientific meetings and events sponsored by NCI and other scientific organizations, is available at: http://calendar.cancer.gov/
|
|
On March 29, Dr. John D. Potter of the Fred Hutchinson Cancer Center presented a Grand Rounds lecture, "Toward the Last Cohort," in the Clinical Center's Lipsett Amphitheater.
Dr. Potter presented a proposal for a large cohort study that aims, among other things, to characterize the genetic variation and environmental exposures involved in different types of cancer and to identify biological markers for the early detection of disease. Another goal is to expand the molecular classification of tumors.
To achieve all this, Dr. Potter said he would need a very large cohort of ethnically diverse individuals who are well characterized genetically, whose exposures are diverse and carefully documented, and whose illnesses and mortality can be monitored.
"A hundred thousand people would not be enough," said Dr. Potter. "A million might be a good start."
This group, "the last cohort," could be used to examine the impact of exposures on the causes and rates of disease and also to study the interaction of these exposures with genetic variation. The project, which would focus on cancer but could be a model for other diseases, would define a "pattern of human disease susceptibility and resistance."
The project would collect biological samples from individuals - to define DNA sequences and protein expression - and information about lifestyle and environmental exposures, including everything from medical history, family history, and reproductive history to data on diet, smoking, hormone use, and exercise.
The exposure data are key, Dr. Potter emphasized. He said that there will be remarkable high-throughput gene sequencing and proteomics capabilities in the next 5 or 10 years, but there is also a risk for not having the infrastructure in place to collect the data on exposures, thereby compromising our ability to use the combination of information to understand and treat disease.
One goal of the cohort study would be to continue the recent progress in developing a system for the molecular classification of tumor subtypes and creating more precise definitions of disease, or phenotypes. In general, the current system for characterizing disease runs from precise molecular classifications of cancer to vague heterogeneous syndromes such as schizophrenia, Dr. Potter said.
"The disease classification system based on histology that has served us well since the 19th century is beginning to fall apart because it isn't helping us understand diseases and how to treat them," he said. "The good news," he added, "is that you can always improve the classification system as new information becomes available."
Dr. Potter said that the study offers the potential for discovering biological markers for early detection because individuals would be followed for a prolonged period of time and would donate blood and other bodily fluids at regular intervals. By the time patients are typically diagnosed and recruited for studies, most indicators of early-stage disease have come and gone.
Dr. Potter also explained that the variation in cancer rates among populations around the world cannot be accounted for by variation in genes alone. Rather, it must be due to differences in environmental exposures or to differences in the interactions between genes and exposures.
See also: "Toward the Last Cohort," an editorial in the June 2004 Cancer Epidemiology Biomarkers & Prevention.
|
 |
|