| Index | Site Map | FAQ | Facility Info | Reading Rm | New | Help | Glossary | Contact Us | |||

Program-Specific Guidance About Exempt Distribution Licenses - Final Report (NUREG-1556, Vol. 8)On this page:
Publication InformationManuscript Completed: September 1998 Prepared by Division of Industrial and Medical Nuclear Safety AbstractAs part of its redesign of the materials licensing process, NRC is consolidating and updating numerous guidance documents into a single comprehensive repository as described in NUREG-1539, "Methodology and Findings of the NRC's Materials Licensing Process Redesign," dated April 1996, and draft NUREG-1541, "Process and Design for Consolidating and Updating Materials Licensing Guidance," dated April 1996. NUREG-1556, Vol. 8, "Consolidated Guidance about Materials Licenses: Program-Specific Guidance about Exempt Distribution Licenses," dated September, 1998, is the eighth program-specific guidance developed for the new process and is intended for use by applicants, licensees, and NRC staff. It will also be available to Agreement States. This document updates and supersedes the guidance found in Draft NUREG -1562, "Standard Review Plan for Applications for Licenses to Distribute Byproduct Material to Persons Exempt from the Requirements for an NRC License." Exemptions from the requirements for an NRC license to persons who receive, possess, use, transfer, own, or acquire byproduct material in exempt distribution products, are provided in 10 CFR Part 30, "Rules of General Applicability to Domestic Licensing of Byproduct Material." Exempt distribution products include silicon chips, electron tubes, resins, check sources, carbon-14 urea capsules, gunsights, and smoke detectors are distributed by persons who have a specific license from the Commission authorizing such distribution to persons exempt from the requirements for an NRC license. This document provides assistance to applicants and licensees in preparing license applications. It also describes the methods acceptable to NRC license reviewers in implementing the regulations and the techniques used by the reviewers in evaluating the applications to determine if the proposed exempt distribution activity is acceptable for licensing purposes. The guidance contained herein does not represent new or proposed regulatory requirements, and licensees will not be inspected against any portion of it. Additionally, regulatory compliance with all applicable regulations is not assured by licensees who adopt any portion of, or apply the principles described in, this guidance. FiguresFigure 9.1 Example of Exempt Concentrations: Irradiated Topaz Figure 9.2 Example of Exempt Concentrations: Electron Tube Figure 9.3 Example of Exempt Quantities: Labeling Figure 9.4 Example of Radioactive Drug: Labeling Figure 9.5 Example of Self-Luminous Products: Front and Rear Gunsights ForewordThe United States Nuclear Regulatory Commission (NRC) is using Business Process Redesign (BPR) techniques to redesign its materials licensing process. This effort is described in NUREG-1539, "Methodology and Findings of the NRC's Materials Licensing Process Redesign," dated April 1996. A critical element of the new process is consolidating and updating numerous guidance documents into a NUREG-series of reports. Below is a list of volumes currently included in the NUREG-1556 series.ecific Guidance about Exempt Distribution Licenses," dated September 1998, represents a step in the transition from the current paper-based process to the new electronic process. This document is available on the Internet at the following address: <http://www.nrc.gov/reading-rm/doc-collections/nuregs/staff/sr1556/v8/index.html>.
The current document, NUREG-1556, Vol. 8, "Consolidated Guidance about Materials Licenses: Program-Specific Guidance about Exempt Distribution Licenses," dated September 1998, is the eighth program-specific guidance developed for the new process. It is intended for use by applicants, licensees, NRC license reviewers, and other NRC personnel. It combines and updates the guidance for applicants and licensees previously found in NUREG -1562, "Standard Review Plan for Applications for Licenses to Distribute Byproduct Material to Persons Exempt from the Requirements for an NRC License." NUREG-1556, Vol. 8, "Consolidated Guidance about Materials Licenses: Program-Specific Guidance about Exempt Distribution Licenses," dated September 1998, is not a substitute for NRC regulations, and compliance is not required. The approaches and methods described in this report are provided for information only. Frederick C. Combs, Acting Director AcknowledgmentsThe writing team thanks the individuals listed below for assisting in the development and review of the report. All participants provided valuable insights, observations, and recommendations. The team also thanks Kay Avery, Judy Boykin, Grace S. Lee, D. W. Benedict Llewellyn, Steven W. Schawaroch, and Gina G. Thompson of Computer Sciences Corporation. The ParticipantsBaggett, Steven L. Abbreviations
1 Purpose of ReportThis report provides guidance to an applicant in preparing an exempt distribution license application as well as NRC criteria for evaluating an exempt distribution license application. Exempt distribution licenses authorize the initial distribution of byproduct material to persons exempt from the requirements (exempt distribution) for a Nuclear Regulatory Commission (NRC or Commission) license pursuant to 10 CFR 30.14, 30.15, 30.16, 30.18, 30.19, 30.20, and 30.21. This report identifies the information needed to complete NRC Form 313 (Appendix B), "Application for Material License," for the use of byproduct material contained in products distributed to persons without a license. The information collection requirements in 10 CFR Part 30 and 32 and NRC Form 313 have been approved under the Office of Management and Budget (OMB) Clearance Nos. 3150-0017, 3150-0001, and 3150-0120, respectively. The format within this document for each item of technical information is as follows:
Notes and References are self-explanatory and may not be found for each item on NRC Form 313. 2 Agreement StatesCertain states, called Agreement States (see Figure 2.1), have entered into agreements with the NRC that give them the authority to license and inspect byproduct, source, or special nuclear materials used or possessed within their borders. Any applicant other than a Federal Agency who wishes to possess or use licensed material in one of these Agreement States needs to contact the responsible officials in that State for guidance on preparing an application; file these applications with State officials, not with the NRC. Table 2.1 provides a quick way to check on which Agency has regulatory authority. Table 2.1 Who Regulates the Activity?*
* Except for a few specific instances, NRC has retained the authority to regulate and license the distribution of products containing radioactive material to persons exempt from licensing. See Section 5.2, "Agreement States and Exempt Distribution" for further discussion and clarification. Figure 2.1 U.S. Map. Location of NRC Offices. Reference: A current list of Agreement States (including
names, addresses, and telephone numbers of responsible officials) is available
by choosing "Directories" on the NRC Office of State Programs' (OSP's)
Home Page, <http://www.hsrd.ornl.gov/nrc/home.htm>
All Agreement States Letter, SP-96-022, dated February 16, 1996, is available on OSP's Home Page, <http://www.hsrd.ornl.gov/nrc/home.htm>; choose "NRC-State Communications," then choose "All of the Above," and follow the directions for submitting a query for "SP96022." As an alternative, request the letter from OSP; call NRC's toll free number (800) 368-5642 and then ask for extension 415-3340. 3 Management ResponsibilityThe NRC recognizes that effective radiation safety program management is vital to achieving safe and compliant operations. NRC believes that consistent compliance with its regulations provides reasonable assurance that licensed activities will be conducted safely. NRC also believes that effective management will result in increased safety and compliance.
To ensure adequate management involvement, a management representative must sign the submitted application acknowledging management's commitments and responsibility for the following:
4 Applicable RegulationsThe regulations applicable to persons exempt from the requirements for a license are located in 10 CFR Part 30. Part 32, "Specific Domestic Licenses to Manufacture or Transfer Certain Items Containing Byproduct Material" outlines, in part, the information required to be submitted for a specific license to apply or incorporate byproduct material into a product; to initially transfer for sale or distribution products containing byproduct material; or to manufacture, possess, produce, package or repackage products containing byproduct material. The following is a list of the regulations providing the exemptions in Part 30 and the corresponding requirements on the distributor in Part 32. �.14 Exempt concentrations �.11 Introduction of byproduct material into products or materials in exempt concentrations, and transfer of ownership or possession: Requirements for license. �.12 Same: Records and material transfer reports. �.13 Same: Prohibition of introduction. �.15 Certain items containing byproduct material �.14 Certain items containing byproduct material; requirements for license to apply or initially transfer. �.15 Same: Quality assurance, prohibition of transfer, and labeling. �.16 Certain items containing byproduct material: Records and reports of transfer. �.16 Resins containing scandium-46 and designed for sand-consolidation in oil wells �.17 Resins containing scandium-46 and designed for sand-consolidation in oil wells: requirements for license to manufacture, or to initially transfer for sale or distribution. �.18 Exempt quantities �.18 Manufacture, distribution and transfer of exempt quantities of byproduct material: Requirements for license. �.19 Same: Conditions of licenses. �.20 Same: Records and material transfer reports. �.19 Self-luminous products containing tritium, krypton-85, or promethium-147 �.22 Self-luminous products containing tritium, krypton-85, or promethium-147: Requirements for license to manufacture, process, produce, or initially transfer. �.23 Same: Safety criteria. �.24 Same: Table of organ doses. �.25 Conditions of licenses issued under �.22: Quality control, labeling, and reports of transfer. �.20 Gas and aerosol detectors containing byproduct material �.26 Gas and aerosol detectors containing byproduct material: Requirements for license to manufacture, process, produce, or initially transfer. �.27 Same: Safety criteria. �.28 Same: Table of organ doses. �.29 Conditions of licenses issued under �.26: Quality control, labeling, and reports of transfer. �.21 Radioactive drug: Capsules containing carbon-14 urea for "in vivo" diagnostic use for humans �.21 Radioactive drug: Manufacture, preparation, or transfer for commercial distribution of capsules containing carbon-14 urea each for "in vivo" diagnostic use for humans to persons exempt from licensing; Requirements for a license. �.21a Same: Conditions of license. Additional regulations applicable to exempt distribution licenses are found in the following 10 CFR Parts:
To request copies of the above documents, call GPO's order desk in Washington,
DC at (202) 512-1800. Order the two-volume bound version of Title
10, Code of Federal Regulations, Parts 0-50 and 51-199 from the GPO, Superintendent
of Documents, Post Office Box 371954, Pittsburgh, Pennsylvania 15250-7954.
You may also contact the GPO electronically at <http://www.gpo.gov>
5 Exempt Distribution5.1 GeneralExemptions from licensing requirements are based primarily on a determination by the Commission that the exempted classes of products or types of uses will not constitute an unreasonable risk to the common defense or security or to public health and safety. Radiation safety is primarily dependent on safety features built into the sealed source or device or on restrictions on the amount of radioactive material that can be initially distributed. 10 CFR Part 30, "Rules of General Applicability to Domestic Licensing of Byproduct Material," provides such an exemption from the requirements for an NRC license to persons who receive, possess, use, transfer, own, or acquire byproduct material in exempt distribution products such as silicon chips, electron tubes, resins, check sources, gunsights, and smoke detectors. NRC applies regulatory control on the redistribution of products to persons exempt from the requirements for a license through specific requirements on distributors, as defined in Subpart A, 10 CFR Part 32. Generally, distribution of byproduct material to persons exempt from Regulatory authority can only be made by persons who have a specific license from the Commission authorizing such distribution to persons exempt from the requirements for an NRC license. Except as provided in �.18(a), these exemptions do not apply to persons who manufacture, process, produce, incorporate byproduct material into, initially transfer for sale, or distribute products containing byproduct material. Those engaged in these activities must be licensed in order to initially transfer or distribute to persons exempt from licensing. The distributor is required to ensure to the Commission that all products are manufactured, tested, and distributed in accordance with the specifications provided in its license application. These specific licenses are issued by the Commission and are referred to as "exempt distribution" or "E" licenses. Persons authorized to initially transfer or distribute to persons exempt from licensing must have a license authorizing the possession or use of radioactive material from NRC or an Agreement State before an exempt distribution license will be issued (Section 5.3 Possession Licenses). 5.2 Licensing and RegistrationApplicants are required to provide specific information about the sources and products as outlined in Ё32.11, 32.14, 32.17, 32.18, 32.21, 32.22, and 32.26 concerning the radionuclides and activities, containment and construction, labeling, quality control and assurance programs, etc. NRC will evaluate the information submitted in the application to ensure it meets all applicable standards and regulations and will contact the applicant, if necessary, to obtain additional clarification or information. A sealed source and device (SSD) safety evaluation will be performed by the Materials Safety Branch (MSB) on the sealed sources and devices distributed pursuant to Ё32.22 and 32.26. The device evaluations will contain a review of the type and quantity of byproduct material; the chemical and physical form of the byproduct material; the solubility in water and body fluid of the byproduct material; the details of construction and design of the product; the degree of access of human beings to the product; the expected useful life of the product; the labeling of the product and point-of-sale package; the prototype testing procedures and results; the required safety criteria; the QA/QC procedures; and the proposed uses. Upon completion of the SSD evaluation, a registration certificate (Appendix C) will be issued. The registration certificate, including cover letter to the applicant, will be returned to the license reviewer to review any remaining items necessary to issue the license. After the issuance of a license, licensees must conduct their programs for the manufacture and/or distribution of exempt distribution products in accordance with the following:
Section 30.9 of 10 CFR Part 30, "Rules of General Applicability to Domestic Licensing of Byproduct Material," requires that the information provided in the application be complete and accurate in all material respects. Information is considered to be material if it is likely to change or affect an Agency decision on issuing the license. Therefore, information should be clear, specific, and accurate. Section 30.10, 10 CFR Part 30, "Deliberate misconduct," states that those providing information concerning a licensee's activities may not deliberately engage in misconduct or provide incomplete or inaccurate information to the NRC. It is important that applicants and licensees understand that the information provided in an application and approved in the license is considered a limitation by NRC on the licensee to engage only in those activities and products as described in the application or license. NRC should be notified of any changes or additions to the information submitted in the application. While some changes may not result in an amendment to the license, licensees should not assume that an amendment is not needed or an amendment request has been granted until they receive a written confirmation in the form of a letter or license amendment. Agreement States and Exempt DistributionAgreement States may also license the distribution of exempt concentrations as listed in � 30.70, Schedule A, for applicants within their jurisdiction, and exempt distribution products containing naturally occurring radioactive material not regulated by the NRC. In accordance with Ё150.15(a) and (b), persons in Agreement States are not exempt from NRC licensing and regulatory requirements, with respect to the initial transfer of any equipment, device, commodity, or other product containing source material or byproduct material, whose subsequent possession, use, transfer, and disposal by all other persons are exempted from NRC licensing and regulatory requirements. Under �.14, any person is exempt from the requirements for a license to receive, possess, use, transfer, own or acquire products or materials containing byproduct material in concentrations not in excess of those specified in �.70, Schedule A. The introduction of byproduct material into the product or material, such as silicon chips, for transfer to persons exempt from licensing must be performed in accordance with a specific license issued by an Agreement State or NRC pursuant to �.11 or the general license in �0.20. This exemption does not apply to the transfer of byproduct material contained in any food, beverage, cosmetic, drug or other commodity or product designed for ingestion or inhalation by, or application to, a human being. NRC's Headquarters Office issues all exempt distribution licenses except for exempt distribution products that contain naturally occurring radioactive material (NORM). NORM is solely regulated and licensed by the Agreement States; therefore, the distribution of products containing exempt concentrations of NORM is licensed by the Agreement States. 5.3 Possession LicensesExempt distribution licenses only authorize the product(s) to be distributed to persons exempt from licensing and generally do not authorize possession or use of radioactive material by the distributor. Persons who manufacture, process, produce or initially transfer for sale products containing byproduct material must meet the general requirements of �.33 for possession and use of licensed material on Federal property, in an Agreement State, or in any State subject to NRC jurisdiction. They must also be authorized under specific license for the possession and use of byproduct material. Therefore, applicants for exempt distribution licenses may need to file a separate application for a specific license authorizing possession and use of byproduct material, incident to distribution, with the NRC Regional Office or Agreement State for the State in which the material will be possessed and/or used. The four Regions and the Regional Offices' addresses are provided on NRC Form 3 or in 10 CFR Part 20, Appendix D (Figure 2.1). An exempt distribution license cannot be issued until the applicant obtains a possession and use license. 5.4 Types of Exempt DistributionExempt distribution licenses are based on the types of products to be distributed according to the seven categories of exemptions. The following provides the applicable regulation and some examples of products distributed within each exemption category:
5.5 Proprietary InformationLicense applications are available for review by the general public in the NRC Public Document Rooms; therefore, proprietary information (i.e., information not to be disclosed to the public) should not be included in an application unless necessary. Any proprietary or confidential information submitted should be clearly marked by the applicant as "proprietary," "confidential," "restricted," or "is the express property of Company X," following the procedure(s) in 10 CFR 2.790, "Public inspection, exemption, requests for withholding." Failure to follow this procedure may result in disclosure of the proprietary information to the public or substantial delays in processing the application. Applications containing information marked as "proprietary," "confidential," "restricted," or "is the express property of Company X," will be reviewed to determine if this information is necessary to issue the license. If the information is determined unnecessary, it will be returned to the applicant. If such information is necessary to issue the license, it will be reviewed by the NRC's Office of the General Counsel (OGC) to determine if it is indeed proprietary or confidential and should be withheld from public disclosure. If OGC determines that the application or affidavit is deficient, i.e., does not contain the required information as outlined in �790(b)(4), the applicant will be notified that additional information is needed and that the review will continue when the required information is received. Applicants will be informed that NRC must review the information before determining to withhold it from public disclosure and that the review of their request for licensing may continue. However, a license cannot be issued until after receipt, review, and resolution of the request to withhold information. Once OGC has reviewed the application and affidavit and determined whether to withhold the information from public disclosure, the Commission will notify the licensee by letter of its decision and the appropriateness of their �790 affidavit (see Appendix D). Applicants should write "Proprietary Information" on the top and bottom of the front page of each document containing proprietary information. The license reviewer will place a Proprietary Information cover sheet, NRC Form 190, on the document. Note: Additional procedures for the handling of proprietary information can be found in Directive 12.6 (Formerly MC 2101), "NRC Sensitive Unclassified Information Security Program." 5.6 Foreign VendorsForeign vendors are a unique problem for NRC in that NRC has no jurisdiction over the foreign entities. Pursuant to 10 CFR 110.53, "United States address, records, and inspections," foreign vendors or licensees involved in importing and exporting nuclear material and equipment are required to establish an address in the United States where papers may be served, where records can be maintained, and where the NRC can inspect the applicant's activities and records as necessary to accomplish its mission. Therefore, an exempt distribution license will not be issued to a foreign vendor unless the requirements set forth in �0.53 have been satisfied. 6 How to File6.1 Paper ApplicationForm 313Applicants wishing to distribute or initially transfer products containing byproduct material to persons exempt from licensing, should complete NRC Form 313, "Application for Material License" (Appendix B). An application for a distribution license should not contain information concerning the possession and use of radioactive material covered in the possession license. Since items 7 through 11 of NRC Form 313 pertain to possession and use license and are not applicable to the exempt distribution license, applicants should only complete items 1 through 6, 12 and 13 on the application form itself.
Please note that if it is necessary when filing for a license to reference information contained in other licensees' file(s) or registration certificate(s), whether current, retired or inactive, the information should be submitted, in its entirety, as part of the application. Appendix E contains a "new licensee" letter which provides the applicable regulations and requirements for applying for an exempt distribution license. All license applications will be available for review by the general public in NRC's Public Document Rooms. If it is necessary to submit proprietary information, follow the procedure in 10 CFR 2.790. Failure to follow this procedure could result in disclosure of the proprietary information to the public or substantial delays in processing the application. Employee personal information, e.g., home address, home telephone number, social security number, date of birth, and radiation dose information, should not be submitted unless specifically requested by NRC. As explained in the "Foreword," NRC's new licensing process will be faster and more efficient, in part, through acceptance and processing of electronic applications at some future date. NRC will continue to accept paper applications. However, these will be scanned and put through an optical character reader (OCR) to convert them to electronic format. To ensure a smooth transition, applicants are requested to follow these suggestions:
6.2 Electronic ApplicationAs the electronic licensing process develops, it is anticipated that NRC may provide mechanisms for filing applications via diskettes or CD-ROM, and through the Internet. Additional filing instructions will be provided as these new mechanisms become available. The existing paper process will be used until the electronic process is available. 6.3 Where to FileNRC's Headquarters Office issues all exempt distribution licenses except for exempt distribution products that contain naturally occurring radioactive material (NORM) which are solely regulated and licensed by the Agreement States and for those exempt concentrations as described in "Licensing and Registration" for non-NRC states (Section 5.2). Requests for exempt distribution licenses and device safety evaluations of sealed sources or devices are submitted directly by applicants, the Regions or the MSB to the Division of Industrial and Medical Nuclear Safety, Office of Nuclear Material Safety and Safeguards, U.S. Nuclear Regulatory Commission, Washington, DC 20555-0001 (address is also found at the top of NRC Form 313). 7 Application and Annual FeesEach application for which a fee is specified, including applications for new licenses and license amendments, must be accompanied by the appropriate fee. Refer to 10 CFR 170.31, "Schedule of fees for materials licenses and other regulatory services, including inspections, and import and export licenses," to determine the amount of the fee that must accompany your application. NRC will not issue the new license prior to fee receipt. Once technical review has begun, no fees will be refunded; application fees will be charged regardless of the NRC's disposition of an application or the withdrawal of an application. For applicants wishing to distribute items containing byproduct material pursuant to Ё30.19 and 30.20 (self-luminous products and gas and aerosol detectors), that require a device evaluation, the fee categories are 3B for possession and use license, 3H for distribution license, and 9A for the device evaluation. Applicants desiring to distribute items authorized under Ё30.14, 30.15, 30.16, 30.18, and 30.21 (exempt concentrations, certain items containing byproduct material, resins, exempt quantities, and radioactive drugs), that do not require a device evaluation, the fee categories are 3B for possession and use and 3I for distribution. Most NRC licensees are also subject to annual fees; refer to �1,16; the same fee categories that applied to the application, amendment, renewal and registration fees also apply to the annual fees. Consult �1.11 for additional information on exemptions from annual fees and �1.16(c) on reduced annual fees for licensees that qualify as "small entities." Direct all questions about NRC's fees or completion of Item 12 of NRC Form 313 (Appendix B) to the Office of the Chief Financial Officer (OCFO) at NRC headquarters in Rockville, Maryland, (301) 415-7554. As an alternative, call NRC's toll free number (800) 368-5643 and then ask for extension 415-7554. Payment of fees should be mailed along with the application(s) to the Division of Industrial and Medical Nuclear Safety, Office of Nuclear Material Safety and Safeguards, U.S. Nuclear Regulatory Commission, Washington, DC 20555-0001. NRC conducts rulemaking each year to establish the Part 171 annual fees
and to make any necessary changes to the Part 170 licensing and inspection
fees. The proposed changes to the fees are published in the Federal
Register 8 Contents of an ApplicationThe following comments apply to the indicated items on NRC Form 313 (Appendix B). 8.1 Item 1: License Action TypeTHIS IS AN APPLICATION FOR (Check appropriate item)
Check box A if the application is for a new license. Check box B if the application is for an amendment(1) to an existing license, and provide the license number. Check box C if the application is for the renewal1 of an existing license, and provide the license number. 8.2 Item 2: Applicant's Name and Mailing AddressList the legal name of the applicant's corporation or other legal entity with direct control over use of the radioactive material (product). A division or department within a legal entity may not be a licensee. An individual may be designated as the applicant only if the individual is acting in a private capacity and the use of the radioactive material (product) is not connected with employment in a corporation or other legal entity. Provide the mailing address where correspondence should be sent. A Post Office box number is an acceptable mailing address. Note: While a U.S. address is required in order to issue a license, it is acceptable for the licensee's mailing address and the state code in the license number to be based on an address located in Puerto Rico, Canada, and the Virgin Islands. Notify NRC of changes in mailing address; these changes do not require a fee. Note: NRC must be notified before control of the license is transferred or when bankruptcy proceedings have been initiated. See below for more details. NRC Information Notice (IN) 97-30, "Control of Licensed Material during Reorganizations, Employee-Management Disagreements, and Financial Crises," dated June 3, 1997, discusses the potential for the security and control of licensed material to be compromised during periods of organizational instability. 8.2.1 Timely Notification of Transfer ControlPursuant to 10 CFR 30.34(b), licensees must provide full information and obtain NRC's prior written consent before transferring control of the license, or, as some licensees call it, "transferring the license." Transfers of control may be the result of mergers, buy outs, or majority stock transfers. Although it is not NRC's intent to interfere with the business decisions of licensees, it is necessary for licensees to obtain prior NRC written consent. This is to ensure the following:
Appendix F, excerpted from IN 89-25 (Rev. 1), "Unauthorized Transfer of Ownership or Control of Licensed Activities," dated December 7, 1994, identifies the information to be provided about transferring control. 8.2.2 Notification of Bankruptcy ProceedingsSection 30.34(h), 10 CFR Part 30, requires that, immediately following filing of voluntary or involuntary petition for or against a licensee, licensees must notify the appropriate NRC Regional Administrator, in writing, identifying the bankruptcy court in which the petition was filed and the date of filing. Even though a licensee may have filed for bankruptcy, the licensee remains responsible for all regulatory requirements. NRC needs to know when licensees are in bankruptcy proceedings in order to determine whether there are any public health and safety concerns (e.g., contaminated facility). NRC shares the results of its determinations with other involved entities (e.g., trustee) so that health and safety issues can be resolved before bankruptcy actions are completed. See the Notice of Availability (on the inside front cover of this draft report) to obtain copies of Policy and Guidance Directive PG 8-11, "NMSS Procedures for Reviewing Declarations of Bankruptcy," (dated August 8, 1996) and Inspection Procedure (IP) 87103, "Inspection of Material Licensees Involved in an Incident or Bankruptcy Filing." 8.3 Item 3: Address(es) Where Licensed Material Will Be Used or PossessedAn applicant for an exempt distribution license must be an organization with an address in the United States at which it will receive, possess, and perform quality control checks on the products authorized for distribution and maintain records relating to NRC-related activities, and from which it will distribute the items. Specify street address, city, and state or other descriptive address (e.g., on Highway 10, 5 miles east of the intersection of Highway 10 and State Route 234, Anytown, State) for each and every facility used as a location from which distribution will occur. A Post Office Box address is not acceptable; see Figure 8.1. Each point of distribution will be listed on the exempt distribution license. Note: If the addresses listed are in Agreement States, an exempt distribution license will not be issued or amended until a copy of the corresponding possession license has been provided to NRC. An NRC license does not relieve a licensee from complying with other applicable Federal, State, or local requirements (e.g., local zoning requirements or local ordinances requiring registration of radioactive material). 8.4 Item 4: Person to Be Contacted About This ApplicationIdentify the individual who can provide information and answer questions about the application and the product(s) to be distributed, and include his or her telephone number. It is not necessary for the contact person for an exempt distribution license to be the Radiation Safety Officer designated on the possession license or to have authority to make and implement commitments to the NRC, as long as this person is knowledgeable about the products being distributed. The NRC will contact this individual if there are questions about the application. Note: This item is for information only. It is not necessary to provide for review, any information concerning the individual's training or experience. For legal purposes, documents signed by someone other than the original signatory of the application or a management representative, should contain documentation from the applicant/licensee indicating that this individual is authorized to make legally binding commitments on the part of the licensee; otherwise, have another individual who is authorized to sign the document. Notify NRC if the contact person or his or her telephone number changes so that NRC can contact the applicant or licensee in the future with questions, concerns, or information. This notice is for "information only" and does not require a license amendment or a fee. 8.5 Item 5: Radioactive MaterialApplicants should determine what devices or products are to be distributed and provide information about each type of product, a list of the radionuclides (include manufacturer's name and model number, if applicable), the physical form, and the maximum activity of radioactive material that will be used in each source for each product type. Activity may be specified either in terms of becquerels or in terms of curies. For example, the maximum activity per check source is 0.37 gigabecquerels or 10 microcuries of cesium-137. 8.6 Item 6: Purpose(s) For Which Licensed Material Will Be UsedDescribe in general terms the purpose(s) for which the byproduct material will be used (detailed information about the final product to be distributed is discussed in Section 9), for example, an americium-241 foil source to be incorporated into a smoke detection device for distribution to persons exempt from licensing. 8.7 Item 12: FeesThe next two items on NRC Form 313 are to be completed on the form itself. On NRC Form 313, enter the appropriate fee category from 10 CFR 170.31 and the amount of the fee enclosed with the application. Applicants should be aware that they may be responsible for fees in each category applicable to their application or license. Refer to Section 7.00 for more information. NRC will begin review of licensing requests without the proper fees; however, NRC will not issue a new license, amendment, renewal, or registration certificate prior to receipt of the appropriate fee. 8.8 Item 13: CertificationIndividuals acting in a private capacity are required to date and sign NRC Form 313. Otherwise, representatives of the corporation or legal entity filing the application should date and sign NRC Form 313. Representatives signing an application must be authorized to make binding commitments and to sign official documents on behalf of the applicant. As discussed previously in "Management Responsibility," signing the application acknowledges management's commitment and responsibilities for the radiation protection program. NRC will return all unsigned applications for proper signature. Note: It is a criminal offense to make a willful false statement or representation on applications or correspondence (18 U.S.C. 1001). When the application references commitments, those items become part of the licensing conditions and regulatory requirements. 9 Information Required for Specific Types of Distribution Licenses9.1 General InformationExemptions from licensing requirements are based primarily on a determination by the Commission that the exempted classes of products or types of uses will neither constitute an unreasonable risk to the common defense or security or to public health and safety, nor constitute a frivolous use of radioactive material. 9.1.1 Frivolous UseNRC policy discourages the "frivolous" use of radioactive material pursuant to �.19(c) and generally considers that products proposed for distribution should be of some benefit or use to the public. Typically, the use of radioactive material in toys, novelties such as fishing lures, and adornments have been considered to be of marginal benefit. However, as an exception to this policy, in 1987, the Commission did review the issue of gemstones for distribution and by Commission Directive approved the introduction of byproduct material into gemstones for distribution under �.14. 9.1.2 Sealed Source and Device EvaluationsApplicants wishing to distribute products pursuant to Ё32.22 and 32.26 must submit sufficient information concerning the product to demonstrate that the product will meet the safety criteria set forth for that type of product. The submission should include information about the design and construction of the product, prototype testing, labeling, quality control (QA) procedures, safety criteria, etc. These products undergo a sealed source and/or device safety evaluation based on this information prior to the issuance of a license which is performed by the Materials Safety Branch (MSB). NUREG-1556, Vol. 3, "Consolidated Guidance about Materials Licenses: Applications for Sealed Source and Device Evaluation and Registration" may be used by applicants submitting a sealed source or device design for safety evaluation, registration and licensing. Applications requiring device evaluations will be forwarded to the MSB as technical assistance requests. Upon completion of the evaluation and registration, the registration certificate, including cover letter to the applicant and technical assistance request response will be returned to the license reviewer for review. 9.1.3 Quality Assurance/Quality Control ProgramsQuality control (QC) procedures to be followed in the fabrication of the product and the QC standards the product will be required to meet, are required to be submitted for products under Ё32.14, 32.22, and 32.26. Applicants should develop and implement a QC program that will ensure that the product is manufactured in accordance with the information and representations made in the application. At a minimum, the QC program should meet the specifications similar to those provided in Appendix C, "Quality Control Program Specifications for Certain Exempt Products," Regulatory Guide 6.9, "Establishing Quality Assurance Programs for the Manufacture and Distribution of Sealed Sources and Devices Containing Byproduct Material." Applicants may submit a Quality Assurance (QA) program instead of or in conjunction with a QC program. The QA program should provide control over all activities applicable to the design, fabrication, inspection, testing, maintenance, repair, modification, and distribution of the devices that contain byproduct material. NUREG-1556, Vol. 3, also provides information necessary to establish and implement a QA program that encompasses all of the QA and QC requirements necessary for the manufacture and distribution of sealed sources and devices. Applicants should note that the information in this NUREG is not a substitute for developing and implementing an effective program for the manufacture and distribution of exempt distribution products. However, if an application incorporates by reference procedures in this or any other guidance document, then those procedures become a part of the license conditions and regulatory requirements. For example, if an application or license amendment states that "the manufacturer will follow the acceptance sampling requirements for removable contamination and design conformity as outlined in NUREG-1556, Vol. 3," then the licensee must adhere to the specifications contained in the referenced document. Current practice allows acceptance of the submission of a QA program in lieu of a QC program because the QA program puts more emphasis on the overall management structure and on the program that covers construction of the device from the time of initial design through distribution. Use of a QA program allows for oversight when manufacturing is performed by foreign vendors where the licensee (US distributor) is required to provide documentation and procedures concerning the foreign vendor's QA program; how the licensee will audit the vendor's operations; and the licensee's QA program for final inspection of the product before distribution. 9.1.4 Product Transfer ReportsLicensees are required to file a report concerning the kinds and quantities of byproduct material or products transferred within 30 days following: five years since the preceding report, filing for renewal, and notification of termination of the license pursuant to Ё32.13, 32.16, 32.20, 32.25, and 32.29. 9.2 10 CFR 32.11: Exempt ConcentrationsUnder 10 CFR 30.14, persons are exempt from licensing requirements if the byproduct material contained in a product or material in concentrations not in excess of those specified in �.70, Schedule B, is introduced into the product or material or transferred by a licensee holding a specific license issued pursuant to �.11, "Introduction of byproduct material in exempt concentrations into products or materials, and transfer of ownership or possession: Requirements for license." This means that the person who introduces byproduct material into a product or material, such as silicon chips or wafers, or who initially transfers such a product, must have a specific license authorizing distribution to persons exempt from licensing. The prohibition against the introduction of byproduct material into a product or material if there is knowledge or reason to believe that the product will be distributed or transferred to persons exempt from the requirements for a license under �.14, is found in �.13, "Same: Prohibition of introduction." To obtain authorization to distribute to persons exempt from the requirements, the manufacturer or distributor of the product must provide the required information as outlined in Ё 32.11, 32.12, and 32.13 to evaluate the products before issuance of a license. Appendices G and H contain specific information needed from importers and domestic reactors to support applications for license, pursuant to 10 CFR 32.11, to distribute neutron-irradiated gems to persons exempt from licensing (Figure 9.1). Figure 9.1 Example of Exempt Concentrations: Irradiated Topaz Note: The introduction of exempt concentrations of byproduct material for most �.14 products can be authorized by the Regions or Agreement States as a line item on the possession license. However, because the introduction for gemstones is accomplished by the use of a reactor and the Agreement States do not have the authority to regulate reactors, distributors of gemstones must obtain an exempt distribution license from NRC headquarters. Appendix I contains a checklist for NRC's use in reviewing exempt distribution product license applications. 9.3 10 CFR 32.14: Certain Items Containing Byproduct MaterialUnder 10 CFR 30.15, persons who apply or incorporate byproduct material into or who initially transfer or distribute products such as electron tubes, watches with luminous paint, or ionizing radiation measuring instruments containing calibration sources to persons exempt from licensing, must have a license pursuant to �.14, "Certain items containing byproduct material; requirements for license to apply or initially transfer." The product information as outlined in Ё 32.14, 32.15, and 32.16 must be provided for review in order to obtain an exempt distribution license. Note: For those products requiring labeling, NRC's policy is that the smallest item distributed must display the required label. If this is not possible, then the label should be placed as close as possible to the product. For example, if an electron tube is too small to label, then the label should be placed on the next smallest container, such as the bubble pack containing the electron tube. For electron tubes, lamps, etc. (Figure 9.2), applicants can use mathematical calculations or functionality tests to demonstrate and verify that each product contains no more than the quantity of byproduct material specified for that product, pursuant to �.14(c). The functionality tests may involve testing each tube or lamp to confirm that it works and that the light output is within the range known for that tube or lamp for which the specific activity has been determined. Non-working product or below par output are considered indicative of leaking tubes. Figure 9.2 Example of Exempt Concentrations: Electron Tube. For watches, applicants should determine and submit the appropriate methods of prototype testing to demonstrate and verify, for that type of product, that the method of containment or binding of the byproduct material in the product is such that the radioactive material will not be released or be removed from the product under the most severe conditions that the product is likely to encounter under everyday normal use and handling. Tritium will be considered to be properly bound to dials, hands, and pointers if there is no visible flaking or chipping or if the light sources have not become loosened or detached from the dials, hands, or pointers; and the total loss of tritium does not exceed 5 percent of the total tritium when prototype dials, hands, and pointers are subjected to prototype testing. The types of prototype tests used for painted watches might include vibration, bending, and immersion, whereas the bending test might be substituted with a vibration or shock test for watches containing glass vials. Applicants may reference Appendix O, "Standard Requirements for Tritium Illuminated Gunsights Containing Tritium Gas Sealed in Glass Vials," for descriptions of acceptable prototype test methods for tritium gas in glass vials. Appendix J contains a checklist for NRC's use in reviewing exempt distribution product license application. 9.4 10 CFR 32.17: Resins Containing Scandium-46 And Designed For Sand-consolidation in Oil WellsUnder 10 CFR 30.16, persons who manufacture or initially transfer or distribute resins containing scandium-46 to persons exempt from licensing must be licensed pursuant to � 32.17 or equivalent regulations of an Agreement State and must submit for review the product information as required in this section. Appendix K contains a checklist for NRC's use in reviewing exempt distribution product license applications. 9.5 10 CFR 32.18: Exempt QuantitiesSection 32.18(a), 10 CFR Part 32, "Manufacture, distribution and transfer of exempt quantities of byproduct material: Requirements for license," authorizes an exemption to persons who receive, possess, use, transfer, own, or acquire byproduct material in individual quantities not exceeding limits set in �.71, Schedule B. This exemption allows persons to receive, possess, use, own, or acquire small quantities of byproduct material and to transfer items such as tissue samples and counting standards to other unlicensed persons on an occasional basis, not for commercial benefit, without a distribution license. Figure 9.3 Example of Exempt Quantities: Labeling When the transfer of byproduct material in individual quantities not exceeding the limits set in �.71, Schedule B, occurs for commercial benefit, then Ё30.18(c) and (d) apply and the manufacture, transfer, or distribution to persons exempt from licensing requirements must be specifically licensed. Therefore, each person engaging in the commercial transfer or distribution of exempt distribution products must have a license authorizing distribution under �.18. The commercial transfer of a product refers to the introduction of a material into the marketplace, whether or not a charge is assessed for that distribution. Commercial benefit does not necessarily include a monetary exchange. Those persons wishing to distribute byproduct material in individual quantities not in excess of those listed in �.71, Schedule B, for commercial benefit, such as check sources and calibration standards, to persons exempt from licensing must submit information about the product as outlined in Ё 32.18, 32.19, 32.20. The submission should identify the byproduct material to be used and the type(s) (e.g., disc check sources, rod sources, scintillation counting standards) of products intended for distribution and provide a drawing (or picture) of the product type(s). The drawing should indicate the location of the required label. Applicants should request authorization only for the isotopes which are of interest. When appropriate, it is acceptable to reference the byproduct material as: all isotopes not to exceed the activities listed in �.71, Schedule B. The application should clearly state the form, chemical and physical, of the byproduct material and confirm that its intended use is for its radioactive properties and that it is not to be incorporated into any manufactured or assembled commodity, product, or device intended for commercial distribution. The applicant should confirm that the named isotopes are not to be contained in any food, beverage, cosmetic, drug, or other commodity (product) designed for ingestion or inhalation by, or in an application to, a human being nor incorporated into any device. Applications should contain copies of the required labels and product brochures to be distributed with the product. The submission of "generic" labels or a statement (Figure 9.3) indicating that the required information will be contained on the label will be acceptable provided the required information remains as submitted and meets the necessary requirements. This allows licensees to change information on the labels such as brand names or telephone numbers without having to amend their license. For example, the licensee may state that the label on the check sources will contain, at least, the following: the words "Radioactive Material" Radioisotope: XXXX, and Activity: XXX microcuries. In addition to the required statements in �.19(c) and (d), either the label or accompanying product brochure must contain additional basic radiation safety and good laboratory practices and instructions pertaining to the proper handling, use, storage, and disposal of the radioactive material (see example in Appendix L). The information included in the product brochure should be appropriate to the product and its use. Products authorized for exempt distribution are generally received by persons exempt from the requirements for a license. However, if the customer receiving the exempt quantity is a specific licensee, then the customer is subject to the requirements of 10 CFR Part 20 in areas where 10 CFR 30.18 is silent (e.g. waste disposal). Therefore, the information provided to the licensee's customers should not imply regulatory restrictions. For example, statements in the product brochures that the products must be disposed of in a certain manner or returned to the licensee, etc., are inappropriate and should not be contained in the information provided to the licensee's customers, except for those customers that are licensed for those particular radionuclides. Appendix L provides an example of a product brochure to accompany an exempt quantity product that is acceptable for customers that are also licensees. Appendix M contains a checklist for NRC's use in reviewing exempt distribution product license applications. 9.6 10 CFR 32.21: Radioactive Drug: Capsules Containing Carbon-14 Urea For "In Vivo" Diagnostic Use For HumansSection 30.21, 10 CFR Part 30 provides an exemption from the requirements for a license for persons who receive, possess, use transfer, own, or acquire capsules containing one microcurie of carbon-14 urea for "in vivo" diagnostic use for humans. Persons who desire to manufacture, prepare, process, produce, package, repackage, or transfer for commercial distribution such capsules must obtain a specific license authorizing distribution to persons exempt pursuant to �.21. The use of carbon-14 urea capsules for research involving human subjects is prohibited under this exemption and requires that a specific license pursuant to 10 CFR Part 35 be obtained for this purpose. Applications should contain evidence confirming that the applicant meets the requirements under �/strong>32.72(a)(2) requiring that the applicant is either registered or licensed with the U.S. Food and Drug Administrations or with a state agency as a drug manufacturer; or licensed as a pharmacy by a State Board of Pharmacy; or operating as a nuclear pharmacy within a Federal medical institution. The application should indicate that the product is in the form of a capsule, identified as radioactive, and to be used for its radioactive properties, but is not incorporated into any manufactured or assembled commodity, product, or device intended for commercial distribution. The applicant should confirm that the carbon-14 urea is not contained in any food, beverage, cosmetic, drug (except as described in this section), or other commodity designed for ingestion or inhalation by, or topical application to, a human being. The application should contain copies of the prototype labels (Figure 9.4) and brochures for NRC approval. Figure 9.4 Example of Radioactive Drug: Labeling Appendix N contains an NRC checklist for NRC's use in reviewing exempt distribution product license application. 9.7 10 CFR 32.22: Self-Luminous Products Containing Tritium, Krypton-85, or Promethium-147Under 10 CFR 30.19, persons are exempt from the requirements for a license provided the products, such as gunsights (Figure 9.5) and watches, have been initially transferred or distributed in accordance with a license issued pursuant to �.22. The information to be submitted is outlined in Ё 32.22, 32.23, 32.24, and 32.25, "Self-luminous products containing tritium, krypton-85 or promethium-147: Requirements for license to manufacture, process, produce, or initially transfer." The products authorized under �.22 undergo a sealed source and/or device safety evaluation prior to the issuance of a license (see Section 9.1.2). Figure 9.5 Example of Self-Luminous Products: Front and Rear Gunsights Applicants should list all models of each type of product they wish to distribute. Applicants may request to have a model listed as a series. In order to have the model listed as a series, there should be similarities in the design and construction of the devices. Applicants should provide detailed engineering drawings of each basic device series containing the overall dimensions, the minimum and maximum dimensions for each series type, the tolerances, description or identification of the construction materials, and the source mounting configuration(s) to be used with each series type. This information must be provided for each type of material used such as steel, aluminum, or plastic. The submission should include a list of the differences between the models in that series. Appendix O, "Standard Requirements for Gunsights Containing Tritium Gas Sealed in Glass Vials," should be used in preparing the license application. All basic model types may be registered as a series based on such differences as size, construction, and source activity. For example, the Series 100 is a "2-Dot" sight and the basic Series 200 is a "2-Bar" sight, and the Series 300 has a larger source activity per tritium source than either the 100 or 200 series. Models contained within each series may be the same basic model within the overall dimensions and tolerance ranges. For example, a basic model designated as the Series 100 with a minimum steel thickness of 0.01 to 0.02 steel thickness is registered to include the 100A model with a 0.01 minimum steel thickness and the 100B with a .018 minimum steel thickness. Note: NRC's policy on labeling is that the smallest item distributed must contain the required label. If this is not possible, then the label should be placed as close as possible to the product. For example, if a gunsight containing tritium is too small to etch the label into, then the label should be placed on the actual gun as close to the gunsight as possible. Acceptable procedures for testing for leaking tritium vial sources may include swipe testing as well as brightness, light output, or immersion testing based on the rates of tritium leakage over a specific time period. Appendix P contains a checklist for NRC's use in reviewing exempt distribution product license applications. 9.8 10 CFR 32.26: Gas and Aerosol Detectors Containing Byproduct MaterialUnder 10 CFR 30.20, persons are exempt from the requirements for a license provided the products, such as smoke detectors (Figure 9.6), have been initially transferred or distributed in accordance with a license issued pursuant to �.26. The product information to be submitted is outlined in Ё 32.26, 32.27, 32.28, and 32.29, "Gas and aerosol detectors containing byproduct material: Requirements for license to manufacture, process, produce, or initially transfer." The products authorized under �.26 undergo a device safety review and evaluation prior to the issuance of a license (see Section 9.1.2). Applicants may reference NUREG/CR-1156, "Environmental Assessment of Ionization Chamber Smoke Detectors Containing Am-241," for additional information concerning smoke detectors. Applicants should list all models of each type of product they wish to distribute. Applicants may request to have a model listed as a series. In order to have the model listed as a series, there should be similarities in the design and construction of the devices. Applicants should provide detailed engineering drawings of each basic device in each series with a list of the differences between the models in that series. The drawings should clearly show all dimensions and tolerances, describe or identify the construction materials, and provide the details of the source mounting. All basic models may be registered as a series based on such differences as size, construction, and source activity. For example, the Series 200 is larger in size than the basic Series 100, and the Series 300 has a larger source activity than either the 100 or 200. Models contained within each series are the same basic model with cosmetic differences, such as lights and timers. For example, a basic model designated as the Series 100 is registered to include the 100A model with a three second timer, and the 100B with a five second timer. While it is not necessary that a sample of the actual smoke detector(s) be provided, applicants should submit a sample or drawing of the typical or generic label and point-of-sale package showing how the requirements of �.29(b) will be met. Appendix Q contains a check list for NRC's use in reviewing exempt distribution product license applications. 10 Deficiency in the ApplicationIf in the process of evaluating an application, it is determined that insufficient information has been submitted, the license reviewer will contact the applicant to obtain the necessary information. Depending on the type and complexity of the information needed, the reviewer may request the additional information through a formal written request or, especially for simple answers and clarifications, via telephone or electronic mail. Applicants may request an extension of time in order to respond to any correspondence or request for additional information about its application provided it is determined that there is good cause and the additional time is reasonable. The request may be in writing or via the telephone. Typically, the reviewer notifies the applicant by telephone that an extension has been granted with the new proposed date. 11 Issuance of a LicenseLicenses authorized pursuant to Ё 32.11 (except for gemstones and silicon chips), 32.14, 32.17, and 32.18 are prepared using a letterhead format (Appendix R) and licenses authorized under Ё 32.11 (gemstones and silicon chips), 32.22, and 32.26 are prepared using NRC Form 374 (Appendix S). All licenses include the following:
The license also contains a general condition which commits the licensee to conducting its program in accordance with the statements, representations, and procedures contained in the documents, including any enclosures, submitted by the applicant. 12 Amendments and Renewals to a LicenseAfter the issuance of a license, licensees must conduct the licensed activities for the manufacture and/or distribution of exempt distribution products in accordance with:
It is the licensee's obligation to keep the license current and anticipate the need for a license amendment as far as possible. If any of the information provided in the original application is to be modified or changed, the licensee must submit an application for a license amendment before the change takes place. Also, to continue the license after its expiration date, the licensee must submit an application for a license renewal at least 30 days before the expiration date (10 CFR 2.109, 10 CFR 30.36(s)). Applications for license amendment, in addition to the following, must provide the appropriate fee. For renewal and amendment requests applicants must do the following:
For renewals, with the exception of those licensed under �.21, file a report with NRC providing information on the products transferred to other persons per their exempt distribution license. The specific information to be included in the product transfer report is outlined for each category of exempt product in the corresponding regulations (Ё 32.12, 32.16, 32.20, 32.25, and 32.29). In requesting renewal of a license, licensees may do the following:
Applications for license renewal filed at least 30 days before the expiration date of the license will receive a "Deemed Timely" (Appendix T) letter confirming that the application has been timely filed and the present license will remain in effect until the NRC takes final action on the renewal application. A copy of this letter should be maintained until the amended license is received. If a renewal application is not received by NRC before the expiration date, the licensee will be without a valid license when the license expires. If the license expires, exempt distribution activities are no longer authorized, and the licensee must cease all distribution activities until a new license can be obtained. Once the license expires, the licensee must submit an application package for a new license. Licensees not wishing to renew their distribution license should send a letter to the NRC before the expiration date of the license with a request that the license be terminated (see Section 14).
Note: Amending or changing the exempt distribution license may also require an amendment of the device registration sheet for additions, deletions, or modifications to models of sealed sources or devices to be distributed. 13 Applications for ExemptionsVarious sections of NRC's regulations address requests for exemptions (e.g., 10 CFR 19.31, 10 CFR 20.2301, 10 CFR 30.11(a)). These regulations state that NRC may grant an exemption, acting on its own initiative or on an application from an interested person. Key considerations are whether the exemption is authorized by law, will endanger life or property or the common defense and security, and is otherwise in the public interest.
Exemptions are not intended for large classes of licenses, and they are generally limited to unique situations. Exemption requests should be accompanied by descriptions of the following:
14 Termination of ActivitiesPursuant to 10 CFR 30.36, exempt distribution licensees may request termination of their NRC license at any time. Licensees should notify NRC within 60 days of its decision to permanently cease licensed activities or the lack of licensed activities for 24 months. Exempt distribution licensees that intend to terminate their possession and use activities, as well, are also responsible for notifying the appropriate NRC or Agreement State authorities concerning the disposition of the possession license and all radioactive material, and for providing records of deposit, etc. to NRC or the Agreement State. Note: A license is not terminated until NRC takes action to terminate the license; therefore, an application for license termination does not relieve the licensee from its obligations to comply with NRC regulations and the terms and conditions of the license, until such time as the license is terminated in writing by NRC. Note: All categories of exempt distribution licenses, except 32.21, require that product transfer reports be filed when discontinuing activities authorized under the license for the period of time between the filing of the preceding report and the request to terminate the license. If no transfers of byproduct material have taken place, then the report should so indicate. Appendix A
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Document Identification | Title | Date |
|---|---|---|
| Draft NUREG-1562 | Draft NUREG -1562, "Standard Review Plan for Applications for Licenses to Distribute Byproduct Material to Persons Exempt from the Requirements for an NRC License." | 12/88 |
| NUREG-1556, Volume 3 | "Consolidated Guidance About Materials Licenses, Applications for Sealed Source and Device Evaluation and Registration." | 10/84 |
| NO.: | NR-***-D-***-E | DATE: | |||
| DEVICE TYPE: Smoke Detector/Gunsight | |||||
| MODEL: ABC | |||||
| MANUFACTURER/DISTRIBUTOR: | Name | ||||
| Street | |||||
| City, State Zip | |||||
| (if manufacturer and distributor are the same, keep subheading as shown. If different, delete the word manufacturer from the subheading) | |||||
| MANUFACTURER: | Name | ||||
| Street | |||||
| City, State Zip | |||||
| (this subheading and information is not necessary if manufacturer and distributor are the same.) | |||||
| SEALED SOURCE MODEL DESIGNATION: | ACME Model 123 | ||||
| ISOTOPE: | MAXIMUM ACTIVITY: | ||||
| Americium-241 | 1.0 microcurie (37 kBq) | ||||
| Hydrogen-3 | 60 millicuries (2.2 GBq) | ||||
| LEAK TEST FREQUENCY: | Not Required | ||||
| PRINCIPAL USE: | (P) Ion Generator, Smoke Detectors | ||||
| (W) Self-Luminous Light Sources | |||||
| CUSTOM DEVICE: | __ | Yes | X | No | |
| DEVICE TYPE: | Smoke Detector/Gunsight | ||||
| DESCRIPTION: | |||||
| Provide a concise, basic description of the device and if more than one model is registered, provide the differences between models. | |||||
| REFERENCES: | |||||
| The following supporting documents for the Model ABC smoke detectors/gunsights are hereby incorporated by reference and are made a part of this registry document. | |||||
| � 's application dated December 25, 0000, with enclosures thereto. | |||||
| � 's letters dated July 4, 1776, and December 25, 0000, with enclosures thereto. | |||||
| � 's facsimiles dated July 4, 1776, and December 25, 0000. | |||||
| ISSUING AGENCY: | |||||
| U.S. Nuclear Regulatory Commission | |||||
| Date: _____________________ | Reviewer: __________________ | ||||
| Name of 1st Reviewer | |||||
| Date: _____________________ | Concurrence: ________________ | ||||
| Name of 2nd Reviewer | |||||
[Applicant Name]
[ATTN: Contact Name]
City, State Zip Code
Dear :
| SUBJECT: | REQUEST FOR WITHHOLDING INFORMATION CONTAINED IN LICENSE APPLICATION |
By NRC Form 313, "Application for Material License," or letter from (Licensee's Name) dated _______, and affidavit dated ________, you submitted proprietary material consisting of client information and requested it be withheld from public disclosure pursuant to 10 CFR 2.790.
You stated that the submitted information should be considered exempt from public disclosure for the following reasons:
1.
2.
We have reviewed your application and the material in accordance with the requirements of �790 and, on the basis of your statements, (Have/Have Not) determined that the submitted information sought to be withheld contains proprietary commercial information.
Therefore, we have determined that the information contained in Items _____ of NRC Form 313 or letter from (Licensee's name) dated _______, marked as proprietary, will be withheld from public disclosure pursuant to �790(b)(5) and Section 103(b) of the Atomic Energy Act of 1954, as amended. Your request for withholding will be maintained by this Office indefinitely or for as long as you continue to hold NRC License No. - - E.
Withholding from public inspection shall not affect the right, if any, of persons properly and directly concerned to inspect the documents. If the need arises, we may send copies of this information to our consultants working in this area. We will, of course, ensure that the consultants have signed the appropriate agreements for handling proprietary information.
If the basis for withholding this information from public inspection should change in the future such that the information could then be made available for public inspection, you should promptly notify the NRC. You should understand that the NRC may have cause to review this determination in the future, for example, if the scope of a Freedom of Information Act request includes your information. In all review situations, if the NRC makes a determination adverse to the above, you will be notified in advance of any public disclosure.
Sincerely,
[Reviewing Official]
[Date]
[Applicant's Name]
[Street/P.O. Box]
[City, State Zip]
Dear [ ]:
This refers to our recent conversation concerning the application process for obtaining a license pursuant to Section 32.XX, 10 CFR Part 32, authorizing the distribution of (product).
In order to possess and use byproduct material, you must first satisfy the general requirements of 10 CFR 30.33. It is my understanding that you will be manufacturing and distributing from (Agreement State) therefore, you must apply for and obtain a specific license authorizing possession and use of byproduct material from the State of [ ] by contacting:
[Agreement State Contact]
[Department Name]
[Street/P.O. Box]
[City, State Zip]
Tele: [ ]
Fax: [ ]
As an applicant wishing to distribute or initially transfer products containing byproduct material, such as (product), to persons exempt from licensing, you must also obtain an exempt distribution license from the U.S. Nuclear Regulatory Commission. (Prior to licensing your (product) for distribution, it will be necessary for our Sealed Source Safety Section to perform a device safety review prior to the issuance of a device registration sheet.) The product information to be submitted for your distribution license is outlined in 10 CFR Part 32, specifically in Sections 32.XX, 32.XX, and 32.XX, and in NUREG-1556, Vol. 8, "Standard Review Plan for Applications for Licenses to Distribute Byproduct Material to Persons Exempt from the Requirements for an NRC License" (Enclosure 1). While it is not necessary that you provide a sample of the (product), you should submit detailed drawings of the device and an example of the point-of-sale package.
Your application for a distribution license should not contain information concerning the possession and use of radioactive material as covered in your possession license therefore, you should only answer questions 1 through 6, and 12 and 13 on the enclosed NRC Form 313, "Application for Material License."
If you have questions or concerns regarding the fees required, you should contact the License Fee and Accounts Receivable Branch at (301) 415-7544 for fee information. Payment of the fee should be mailed with the application package to the U.S. Nuclear Regulatory Commission, Office of Nuclear Material Safety and Safeguards, Division of Industrial and Medical Nuclear Safety, Washington, DC 20555.
Please note that this fee is only for a distribution license (and device registration).
For your use in applying for a distribution license (and device registration), I have enclosed reference copies of 10 CFR Parts 2, 20, 30, 32, 110, 170, and 171.
If I can be of further assistance, please contact me at (301) 415-XXXX.
Sincerely,
[License Reviewer]
| Enclosures: | 1. NUREG-1556, Vol. 8 |
| 2. NRC Form 313 | |
| 3. 10 CFR Parts 2, 20, 30, 32, 110, 170, and 171 |
Licensees must provide full information and obtain NRC's prior written consent before transferring control of the license; some licensees refer to this as "transferring the license." Provide the following information, excerpted from IN 89-25, Rev. 1, "Unauthorized Transfer of Ownership or Control of Licensed Activities," concerning changes of control by the applicant (transferor and/or transferee, as appropriate). If any items are not applicable, so state.
1. The new name of the licensed organization. If there is no change, the licensee should so state.
2. The new licensee contact and telephone number(s) to facilitate communications.
3. Any changes in personnel having control over licensed activities (e.g., officers of a corporation) and any changes in personnel named in the license such as radiation safety officer, authorized users, or any other persons identified in previous license applications as responsible for radiation safety or use of licensed material. The licensee should include information concerning the qualifications, training, and responsibilities of new individuals.
4. An indication of whether the transferor will remain in non-licensed business without the license.
5. A complete, clear description of the transaction, including any transfer of stocks or assets, mergers, etc., so that legal counsel is able, when necessary, to differentiate between name changes and transferring control.
6. A complete description of any planned changes in organization, location, facility, equipment, or procedures (i.e., changes in operating or emergency procedures).
7. A detailed description of any changes in the use, possession, location, or storage of the licensed materials.
8. Any changes in organization, location, facilities, equipment, procedures, or personnel that would require a license amendment even without transferring control.
9. An indication of whether all surveillance items and records (e.g., calibrations, leak tests, surveys, inventories, and accountability requirements) will be current at the time of transfer. Provide a description of the status of all surveillance requirements and records.
10. Confirmation that all records concerning the safe and effective decommissioning of the facility, pursuant to 10 CFR 30.35(g), 40.36(f), 70.25(g), and 72.30(d); public dose; and waste disposal by release to sewers, incineration, radioactive material spills, and on-site burials, have been transferred to the new licensee, if licensed activities will continue at the same location, or to the NRC for license terminations.
11. A description of the status of the facility, specifically, the presence or absence of contamination should be documented. If contamination is present, will decontamination occur before transfer? If not, does the successor company agree to assume full liability for the decontamination of the facility or site?
12. A description of any decontamination plans, including financial assurance arrangements of the transferee, as specified in 10 CFR 30.35, 40.36, and 70.25. Include information about how the transferee and transferor propose to divide the transferor's assets, and responsibility for any cleanup needed at the time of transfer.
13. Confirmation that the transferee agrees to abide by all commitments and representations previously made to NRC by the transferor. These include, but are not limited to: maintaining decommissioning records required by 10 CFR 30.35(g); implementing decontamination activities and decommissioning of the site; and completing corrective actions for open inspection items and enforcement actions.
With regard to contamination of facilities and equipment, the transferee should confirm, in writing, that it accepts full liability for the site, and should provide evidence of adequate resources to fund decommissioning; or the transferor should provide a commitment to decontaminate the facility before transferring control.
With regard to open inspection items, etc., the transferee should confirm, in writing, that it accepts full responsibility for open inspection items and/or any resulting enforcement actions; or the transferee proposes alternative measures for meeting the requirements; or the transferor provides a commitment to close out all such actions with NRC before license transfer.
14. Documentation that the transferor and transferee agree to transferring control of the licensed material and activity, and the conditions of transfer; and the transferee is made aware of all open inspection items and its responsibility for possible resulting enforcement actions.
15. A commitment by the transferee to abide by all constraints, conditions, requirements, representations, and commitments identified in the existing license. If not, the transferee must provide a description of its program, to ensure compliance with the license and regulations.
Gems such as topaz assume an attractive color when irradiated by neutrons in a reactor. These gems, which are used in jewelry (e.g., rings, pendants), remain radioactive as a result of the neutron irradiation.
Those who commercially import neutron-irradiated topaz into the United States need: (1) a "possession" license issued either by the Nuclear Regulatory Commission (NRC) or an Agreement State, depending on the geographic location of the importer and (2) a "distribution" license issued only by NRC.
"Agreement States" are those states, as shown in Enclosure 1, with which NRC or, previously, the Atomic Energy Commission, has entered into an effective agreement under Subsection 274b of the Atomic Energy Act of 1954, as amended.
The "possession" license will authorize the importer to possess the radioactive material contained in neutron-irradiated gems, and it will permit the importation of the radioactive gems into the United States, in accordance with the provisions of 10 CFR 110.27(a)(3). The "distribution" license will authorize the importer to transfer (i.e., sell) neutron-irradiated gems to persons exempt from licensing, e.g., wholesalers, manufacturing jewelers, retail jewelers. Only the importer is required to have a license.
This document outlines for importers the information needed to support applications for both "possession" and "distribution" licenses. Importers located in non-Agreement States may combine the information for the two types of licenses in one document and submit it to NRC as outlined in Section III of this document. Importers located in Agreement States should follow the instructions in Section IV of this document.
The information that must be included in the application can be categorized as follows:
| A. | Basic Information | ||
| 1. | Specify name of applicant | ||
| 2. | Specify applicant's mailing address | ||
| 3. | Identify the person with detailed knowledge of the application that the NRC staff can contact about the application, giving the person's: | ||
| a. | Name | ||
| b. | Title | ||
| c. | Telephone number | ||
| 4. | Specify the location(s): | ||
| a. | At which gems will be received and possessed | ||
| b. | From which irradiated gems will be distributed to persons exempt from licensing | ||
| c. | At which records pertaining to possession and distribution of irradiated gems will be maintained. | ||
| B. | Background Information | ||
| 1. | Describe the material to be imported, including: | ||
| a. | The type of gems (e.g., topaz) | ||
| b. | Extent to which gems have been processed before irradiation (e.g., cut and polished). Note: Only finished gems which do not require cutting, grinding, or polishing after irradiation will be authorized for distribution to persons exempt from licensing. | ||
| c. | The type(s) and sequence (e.g., neutron-irradiation only; neutron followed by accelerator or gamma irradiation) of irradiation or other treatment (e.g., heat) to which gems have been exposed before they are to be imported | ||
| d. | Where and by whom each irradiation or other treatment is performed. Identify U.S. reactors by name and location; identify foreign reactors by name and country. (Note: gems irradiated with gamma radiation, with accelerator produced radiation, or both, do not contain radioactivity regulated by NRC. Accelerator-produced radioactivity is regulated by the individual states.) | ||
| e. | If gems are exposed to additional irradiation or treatment after importation, for neutron irradiation only, the type(s) and sequence and where and by whom each is performed | ||
| f. | How gems are handled to ensure grouping according to geologic origin of gems and type(s) of irradiation or treatment to which gems have been exposed (significant variations in induced radionuclides will result from differences in gems' origin and type(s) of irradiation or treatment received) | ||
| g. | Identification of all radionuclides with physical half-lives greater than 2 hours (regardless of method of production) induced in gems and classification of each as either a "major" or "minor" radionuclide depending on its contribution to total activity in gems to be distributed to persons who are exempt from licensing | ||
| h. | How the information provided in response to Item B.l.g above was obtained and how NRC can be assured that this information is representative of gems imported in the future | ||
| i. | The requested possession limit determined by multiplying the maximum number of gems to be possessed at one time by the maximum total activity anticipated in any one gem. | ||
| 2. | Describe the handling of gems, including: | ||
| a. | Procedures used to ensure that each irradiated gem is free of removable contamination, including a description of sampling, monitoring, counting, and statistical techniques used, specification of the criteria used to determine when gems are essentially "free of removable contamination," and a description of what will happen to gems exceeding the specified criteria. | ||
| b. | The processing of irradiated gems at the importer's facility and the sequence of these activities (e.g., counting of gems and storage for physical decay; mounting in rings, pendants, or other settings) | ||
| c. | The categories of unlicensed organizations to which irradiated gems will be transferred (e.g., wholesaler; manufacturing jeweler; retail jeweler; individual consumer) | ||
| d. | What will be done with gems whose concentrations exceed the criteria specified in response to Item C.2.e. below (Alternatives include hold in storage for physical decay, transfer to a person specifically licensed to receive them, or disposal as radioactive waste in accordance with the requirements of 10 CFR Part 20 or equivalent regulations of an Agreement State.) | ||
| C. | Information Required by 10 CFR 32.11 | ||
| 1. | Paragraph 32.11(a) requires that the general requirements of 10 CFR 30.33 be satisfied. To comply with this requirement (or equivalent requirements of Agreement States), the applicant will: | ||
| a. | Explain how the facilities and equipment proposed in the application are adequate to protect health and minimize danger to life or property; specifically explain how irradiated gems will be stored and secured against unauthorized removal or, when not stored and secured, will be tended under the constant surveillance and immediate control of a knowledgeable, responsible person on the importer's staff. | ||
| b. | Identify by name the individuals who will be responsible for handling, irradiation, storing, counting, evaluating, and controlling the release of irradiated gems; correlate individuals' names with their responsibilities; and describe the training and experience of each of these individuals that assures protection of the public health and safety. | ||
| 2. | Paragraph 32.11(b) requires that certain information be provided. If information on one or more points has already been provided, reference the previous response by section and item number or provide a complete response. To comply with 10 CFR 32.11(b), the applicant will describe: | ||
| a. | The product or material into which byproduct material will be introduced (see response to B.1.a above) | ||
| b. | The intended use of the byproduct material and the product or material into which it is introduced | ||
| c. | The method of introduction (see response to B.1.c. and e. above) | ||
| d. | Initial concentration of byproduct material in the product or material | ||
| e. | Estimated maximum concentration of the radioisotopes in the product or material at the time of transfer to persons exempt from licensing | ||
| f. | Control methods to assure that no more than the specified maximum concentration is in the product at time of transfer | ||
| g. | Estimated time interval between introduction and transfer of the product or material (i.e., between completion of all types of irradiation and transfer to unlicensed person). | ||
| 3. | Paragraph 32.11(c) requires applicants to provide reasonable assurance of the following: | ||
| a. | Concentrations of byproduct material at time of transfer will not exceed the concentrations in 10 CFR 30.70, Schedule A. | ||
| Note: the limit for a single radionuclide is given in 10 CFR 30.70; the limits for multiple radionuclides are calculated using the "sum of the ratios" method described in Note 2 of 10 CFR 30.70, Schedule A. | |||
| Note: for isotopes not specifically listed in 10 CFR 30.70, Schedule A, the release concentration can be calculated using the formula: | |||
| C = ALI / (3000 x 365) | |||
| where 3000 is the daily water intake for reference man, 365 is days per year and ALI is the annual limit of intake for ingestion. | |||
| b. | Reconcentration of the byproduct material in concentrations exceeding those specified in 10 CFR 30.70 is not likely (e.g., in the case of gemstones, one could consider that neutron-irradiation followed by accelerator-irradiation could increase the induced activity and thus be considered "reconcentration") | ||
| c. | Use of concentrations lower than those specified in response to Item C.2.e. are not feasible (i.e., why maximum values for a single radionuclide should not be lower; why values for multiple radionuclides should not be calculated by setting the "sum of the ratios" equal to a value less than unity) | ||
| d. | The product or material is not likely to be incorporated into any food, beverage, cosmetic, drug, or other commodity or product designed for ingestion or inhalation by a human being. | ||
| D. | Information on Quality Assurance (QA) Program | ||
| Importers must have a QA program. If they do not wish to do the QA themselves, importers may contract this work to another organization. In this case, the contract organization's identity, mailing address, location of work for the importer, etc., must be provided and all responses to the items listed below must clearly explain who (importer or contract organization) will perform each function. When a contract organization is employed to assist an importer (i.e., a licensee), the importer will still be responsible for proper performance of the QA program and must conduct appropriate audits and reviews to ensure that the QA program is being performed as described in the importer's correspondence with NRC. | |||
| 1. | Describe the radiation detection equipment and shielding associated with it that are to be used to identify and quantify the radioactivity induced in gems | ||
| 2. | Specify the frequency, standards (including radionuclide, activity, and accuracy), and procedures used to calibrate such radiation detection equipment | ||
| 3. | Describe counting procedures and how external measurements are converted to concentration values in terms of microcuries per gram. Your description should include, but is not limited to: | ||
| a. | selection of samples; | ||
| b. | maximum and minimum sample size (in terms of number of stones and mass); | ||
| c. | counting efficiency; | ||
| d. | counting times; | ||
| e. | counting geometry; | ||
| f. | time of counting (in relation to completion of irradiation and transfer to unlicensed persons); | ||
| g. | lower limit of detection; | ||
| h. | statistical methods for analyzing data, calculating background and lower level of detection, and determining confidence levels; | ||
| i. | procedures for minimizing "false negatives" (i.e., failure to identify individual gems with radionuclide concentrations greater than those specified in response to Item C.2.e.); and | ||
| j. | sample calculations. | ||
| At a minimum, your procedures must be sufficient to ensure that: | |||
| a. | After each irradiation, measurements performed on gems are adequate to identify all induced radionuclides | ||
| b. | Before release to unlicensed persons, gems are analyzed to ensure that the concentrations listed in 10 CFR 30.70 are not exceeded; because multiple radionuclides will normally be present, the "sum of the ratios" does not exceed unity. (In lieu of use of the "sum of the ratios," it would be acceptable to assure that (1) induced beta and/or gamma emitting byproduct material has a physical half-life less than 3 years and (2) concentration does not exceed 1 x 10-6 礐i/gm. | ||
| c. | If the activity is not quantitatively measured in each gem individually (i.e., if quantitative measurements are made on groups of gems), there is only 1 chance in a 1,000 that an outlier gem will contain more than twice the appropriate 10 CFR 30.70 maximum value (for single or multiple radionuclides). | ||
| 4. | Specify who will be responsible for the QA program, and describe this individual's training and experience in detection and analysis of low-levels of radioactivity. If this individual was identified in response to Item C.l.b, it is not necessary to repeat the individual's qualifications, provided that the response to Item C.l.b. includes a clear description of the person's training and experience in low-level counting techniques. | ||
| 5. | Describe the QA program used to assure reliable data, including: | ||
| a. | The standards, frequency and procedures used to perform constancy tests on the counting systems | ||
| b. | The methods and frequency of introducing "spiked" samples into the routine counting process to assure identification of gems with concentrations in excess of your criteria (i.e., response to Item C.2.e. above). | ||
| 6. | Provide a commitment that, during the term of the license, the applicant will comply promptly with requests from NRC designed to monitor counting techniques. The general nature of these requests is outlined below: | ||
| a. | Upon request, the applicant will provide samples of irradiated gems to NRC for independent verification of radionuclide identity and concentration. NRC's request will be in writing, signed by the appropriate Regional Administrator or the Director, Office of Nuclear Material Safety and Safeguards. The request will specify who (i.e., NRC representative, NRC contractor, or applicant) will select the samples for independent verification. After analysis, samples will be returned promptly to the applicant. | ||
| b. | Upon request, the applicant will analyze qualitatively, quantitatively, or both gems or groups of gems provided by NRC or its contractor. The request will be in writing; signed by the appropriate Regional Administrator or Director, Office of Nuclear Material Safety and Safeguards; will specify the type of analysis requested and techniques to be followed; and will provide instructions for reporting results and for returning gems. | ||
| E. | Information Needed to Support Request for Exemption from Portion of 10 CFR 32.11(c) | ||
| Note that 10 CFR 32.11(c), among other things, prohibits the incorporation of exempt concentrations into products or materials designed for application to human beings. Neutron-irradiated gems with induced activity could be expected to be set in jewelry and worn by consumers, i.e., "applied to human beings." In order to grant licenses authorizing distribution of these gems to unlicensed persons, it will be necessary to grant a limited exemption from the requirements of 10 CFR 32.11(c) as was directed by the Commission. Section 30.11 provides for the granting of exemptions. | |||
| 1. | To fulfill the requirements of 10 CFR 30.11(a), make a specific request for an exemption from that portion of 10 CFR 32.11(c) that prohibits incorporation of exempt concentrations in products or materials designed for application to a human being. Your request may be worded as follows: "If NRC considers gems to be products intended for application to human beings, then an exemption from this portion requirements in 10 CFR 32.11(c) is requested." | ||
| 2. | Using a worst case scenario, calculate the annual radiation dose and assess the health risk to unlicensed persons. Calculate the dose at contact and at 4 cm from jewelry (e.g., pendant) containing neutron-irradiated gems that is worn continuously (24 hours per day, 365 days per year). Assume that these gems contain those radionuclides (identified in your response to Item B.1.g) with the longest physical half-lives and highest energy emissions at the maximum concentrations (identified in your response to Item C.2.e.) you propose to release to unlicensed persons. Dose calculations must consider all types of emissions (e.g., beta, gamma) from the identified radionuclides. | ||
| 3. | Provide similar calculations and assessments for gems that are outliers (i.e., gems with concentrations as much as twice the criteria you plan to use). | ||
Generic Letter 88-4 (dated February 23, 1988) discusses the Nuclear Regulatory Commission's (NRC's) position on distribution of neutron-irradiated topaz to unlicensed persons. As indicated in that Generic Letter and under the authority of Section 81 of the Atomic Energy Act of 1954, as amended, the staff plans to control distribution of these gems at the source and envisions two principal groups of applicants, domestic reactors and commercial importers.
This document outlines for domestic reactors the information needed to support applications for licenses to be issued pursuant to 10 CFR 32.11. The information that must be included in an application can be categorized as follows:
| A. | Basic Information | ||
| 1. | Specify name of applicant | ||
| 2. | Specify applicant's mailing address | ||
| 3. | Identify the person with detailed knowledge of the application that the NRC staff can contact about the application, giving the person's: | ||
| a. | Name | ||
| b. | Title | ||
| c. | Telephone number | ||
| 4. | Specify the location(s): | ||
| a. | At which gems will be irradiated | ||
| b. | From which irradiated gems will be distributed to persons exempt from licensing | ||
| 5. | Specify the docket number of your NRC reactor license. | ||
| 6. | If your reactor is licensed pursuant to 10 CFR 50.21 in Class 104 status, provide information to demonstrate that less "than 50 percent of the annual cost of owning and operating the facility is devoted to the production of materials, products, or energy for sale or commercial distribution, or to the sale of services, other than research and development or education or training." Note: This information will be reviewed with the assistance of the staff of the Office of Nuclear Reactor Regulation. If a license is issued to you pursuant to 10 CFR 32.11, it will require annual submission of similar information. | ||
| B. | Background Information | ||
| 1. | Describe what is to be irradiated, including: | ||
| a. | The type of gems (e.g., topaz) | ||
| b. | Extent to which gems have been processed before irradiation (e.g., cut and polished). Note: Only finished gems which do not require cutting, grinding or polishing after irradiation will be authorized for distribution to persons exempt from licensing. | ||
| c. | Anticipated production (e.g., the estimated maximum number and mass (in grams) of gems to be irradiated at one time and the estimated number of batches per year) | ||
| 2. | Describe how gems are to be irradiated including: | ||
| a. | How gems are handled to ensure grouping according to geologic origin of gems and type(s) of irradiation or treatment to which gems have been exposed (significant variations in induced radionuclides result from differences in gems' origin and type(s) of irradiation received.) | ||
| b. | The type(s) of irradiation and other types of treatment (e.g., heat) and their sequence (e.g., neutron-irradiation only; neutron followed by accelerator or gamma irradiation) | ||
| c. | If gems are subjected to more than one type of irradiation or treatment, where and by whom the other irradiation or treatment is done | ||
| d. | How gems are prepared for irradiation (e.g., the number and mass (in grams) of gems irradiated at one time in one reactor port, type of container used) | ||
| e. | Procedures to be followed for irradiating gems | ||
| f. | The typical irradiation time, neutron energy, and neutron flux and how these are determined | ||
| g. | Identification of all radionuclides with physical half-lives greater than 2 hours (regardless of the method of production) induced in gems and classification of each as either a "major" or "minor" radionuclide depending on its contribution to total activity in gems to be distributed to persons who are exempt from licensing | ||
| h. | How the information provided in response to Item B.2.g. above was obtained and how NRC can be assured that this information is representative of gems to be irradiated in the future. | ||
| 3. | Describe the handling of gems after irradiation, including: | ||
| a. | Procedures used to ensure that each irradiated gem is free of removable contamination, including a description of sampling, monitoring, counting, and statistical techniques used, specification of the criteria used to determine when gems are essentially "free of removable contamination," and a description of what will happen to gems exceeding the specified criteria. | ||
| b. | The post-irradiation processing of irradiated gems at the applicant reactor's facility and the sequence of these activities (e.g., counting of gems and storage for physical decay; mounting in rings, pendants, or other settings; | ||
| c. | The categories of unlicensed organizations to which irradiated gems will be transferred (e.g., wholesaler, manufacturing jeweler, retail jeweler, individual consumer) | ||
| d. | What will be done with gems whose concentrations exceed the criteria specified in response to Item C.2.e. below. (Alternatives include holding in storage for physical decay, transferring to a person specifically licensed to receive them, or disposal as radioactive waste in accordance with the requirements of 10 CFR Part 20.) | ||
| C. | Information Required by 10 CFR 32.11 | ||
| 1. | Paragraph 32.11(a) requires that the general requirements of 10 CFR 30.33 be satisfied. To comply with this requirement, the applicant will: | ||
| a. | Explain how the facilities and equipment proposed in the application are adequate to protect health and minimize danger to life or property with respect to activities to be conducted under this license | ||
| b. | Identify by name the individual who will be responsible for handling, irradiating, storing, counting, evaluating, etc., irradiated gems; correlate individuals' names with their responsibilities; and describe the training and experience of each that ensures protection of the public health and safety. | ||
| 2. | Paragraph 32.11(b) requires that certain information be provided. If information on one or more points has already been provided, reference the previous response by section and item number or provide a complete response. To comply with 10 CFR 32.11(b), the applicant will describe: | ||
| a. | The product or material into which byproduct material will be introduced (see response to B.1 above) | ||
| b. | The intended use of the byproduct material and the product or material into which it is introduced | ||
| c. | The method of introduction (see response to B.2 above) | ||
| d. | Initial concentration of byproduct material in the product or material | ||
| e. | Maximum concentration of the radioisotopes in the product or material at the time of transfer to persons exempt from licensing | ||
| f. | Control methods to assure that no more than the specified maximum concentration is in the product at time of transfer | ||
| g. | Estimated time interval between introduction and transfer of the product or material (i.e., between completion of all types of irradiation and transfer to unlicensed person). | ||
| 3. | Paragraph 32.11(c) requires applicants to provide reasonable assurance of the following: | ||
| a. | Concentrations of byproduct material at time of transfer will not exceed the concentrations in 10 CFR 30.70. Note: that the limit for a single radionuclide is given in 10 CFR 30.70; the limits for multiple radionuclides are calculated using the "sum of the ratios" method described in Note 2 of 10 CFR 30.70. | ||
| b. | Reconcentration of the byproduct material in concentrations exceeding those specified in 10 CFR 30.70 is not likely (e.g., in the case of gemstones, one could consider that neutron-irradiation followed by accelerator-irradiation could increase the induced activity and thus be considered "reconcentration") | ||
| c. | Use of concentrations lower than those specified in response to Item C.2.e are not feasible (e.g., why maximum values for a single radionuclide should not be lower; why values for multiple radionuclides should not be calculated by setting the "sum of the ratios" equal to a value less than unity) | ||
| d. | The product or material is not likely to be incorporated into any food, beverage, cosmetic, drug or other commodity or product designed for ingestion or inhalation by a human being. | ||
| D. | Information on Quality Assurance (QA) Program | ||
| 1. | Describe the radiation detection equipment and shielding associated with it that are to be used to identify and quantify the radioactivity induced in gems | ||
| 2. | Specify the frequency, standards (including radionuclide, activity, and accuracy), and procedures used to calibrate such radiation detection equipment | ||
| 3. | Describe counting procedures and how external measurements are converted to concentration values in terms of microcurie per gram. Your description should include, but is not limited to: | ||
| a. | selection of samples; | ||
| b. | maximum and minimum sample size (in terms of number of stones and mass); | ||
| c. | counting efficiency; | ||
| d. | counting times; | ||
| e. | counting geometry; | ||
| f. | time of counting (in relation to completion of irradiation and transfer to unlicensed persons); | ||
| g. | lower limit of detection; | ||
| h. | statistical methods for analyzing data, calculating background and lower limit of detection, and determining confidence levels; | ||
| i. | procedures for minimizing "false negatives" (i.e., failure to identify individual gems with radionuclide concentrations greater than those specified in response to Item C.2.e.); and | ||
| j. | sample calculations. | ||
| At a minimum, your procedures must be sufficient to ensure that: | |||
| a. | After each irradiation, measurements performed on gems are adequate to identify all induced radionuclides; | ||
| b. | Before release to unlicensed persons, gems are analyzed to ensure that the concentrations listed in 10 CFR 30.70 are not exceeded; because multiple radionuclides will normally be present, the "sum of the ratios" does not exceed unity. In lieu of use of the "sum of the ratios," it would be acceptable to assure that: | ||
| i. induced beta and/or gamma emitting byproduct material has a physical half-life less than 3 years; and | |||
| ii. concentration does not exceed 1 x 10-6 礐i/gm.) | |||
| c. | If the activity is not quantitatively measured in each gem individually (i.e., if quantitative measurements are made on groups of gems), there is only 1 chance in a 1000 that an outlier gem will contain more than twice the appropriate 10 CFR 30.70 maximum value (for single or multiple radionuclides). | ||
| 4. | Specify who will be responsible for the QA program and describe this individual's training and experience in detection and analysis of low-levels of radioactivity. If this individual was identified in response to Item C.1.b, it is not necessary to repeat the individual's qualifications, provided that the response to Item C.1.b includes a clear description of the person's training and experience in low-level counting techniques. | ||
| 5. | Describe the QA program used to assure reliable data, including: | ||
| a. | The standards, frequency and procedures used to perform constancy tests on the counting systems | ||
| b. | The methods and frequency of introducing "spiked" samples into the routine counting process to assure identification of gems with concentrations in excess of your criteria (i.e., response to Item C.2.e. above). | ||
| 6. | Provide a commitment that, during the term of the license, the applicant will comply promptly with requests from NRC designed to monitor counting techniques. The general nature of these requests is outlined below: | ||
| a. | Upon request, the applicant will provide samples of irradiated gems to NRC for independent verification of radionuclide identity and concentration. NRC's request will be in writing, signed by the appropriate Regional Administrator or the Director, Office of Nuclear Material Safety and Safeguards. The request will specify who (i.e., NRC representative, NRC contractor, or applicant) will select the samples for independent verification. After analysis, samples will be returned promptly to the applicant. | ||
| b. | Upon request, the applicant will analyze qualitatively, quantitatively, or both, gems or groups of gems provided by NRC or its contractor. The request will be in writing, signed by the appropriate Regional Administrator or Director, Office of Nuclear Material Safety and Safeguards; will specify the type of analysis requested and techniques to be followed; and will provide instructions for reporting results and for returning gems. | ||
| E. | Information Needed to Support Request for Exemption from Portion of 10 CFR 32.11(c) | ||
| Note that 10 CFR 32.11(c), among other things, prohibits the incorporation of exempt concentrations into products or materials designed for application to human beings. Neutron-irradiated gems with induced activity could be expected to be set in jewelry and worn by consumers, i.e., "applied to human beings." In order to grant licenses authorizing distribution of these gems to unlicensed persons, it will be necessary to grant a limited exemption from the requirements of 10 CFR 32.11(c), as was directed by the Commission. Section 30.11 provides for the granting of exemptions. | |||
| 1. | To fulfill the requirements of 10 CFR 30.11, make a specific request for an exemption from that portion of 10 CFR 32.11(c) that prohibits incorporation of exempt concentrations in products or materials designed for application to a human being. Your request may be worded as follows: "If NRC considers gems to be products intended for application to human beings, then an exemption from this portion of the requirements in 10 CFR 32.11(c) is requested." | ||
| 2. | Using a worst case scenario, calculate the annual radiation dose and assess the health risk to unlicensed persons. Calculate the dose at contact and at 4 cm from jewelry (e.g., pendant) containing neutron-irradiated gems that is worn continuously (24 hours per day, 365 days per year). Assume that these gems contain those radionuclides (identified in your response to Item B.2.g) with the longest physical half-lives and highest energy emissions at the maximum concentrations (identified in your response to Item C.2.e.) you propose to release to unlicensed persons. Dose calculations must consider all types of emissions (e.g., beta, gamma) from the identified radionuclides. | ||
| 3. | Provide similar calculations and assessments for gems that are outliers (i.e., gems with concentrations as much as twice the criteria you plan to use) | ||
| 4. | Submit a copy of the labeling or other information provided to consumers at point-of-sale of neutron-irradiated gems that alerts purchasers of the presence of low-levels of radioactivity so that they can make an informed decision at time of purchase. | ||
| Mail Control No.:_______ | |
| Amendment No.: ________ | Expiration Date: ________ |
| License No.: ________ | Program Code: ________ |
| Docket No.: ________ | Reference No.: ________ |
| Licensee Name: ________________________________________________ | |
| Address: ______________________________________________________ | |
| _____________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ |
Termination ________I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| Mail Control No.: ______ |
| A. | Applicant satisfied general requirements in 30.33, 10 CFR Part 30 | _______ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Provides a description of the product, including: | |
| 1. The product or material into which byproduct material will be introduced | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Intended use of the byproduct material and the product or material into which it is introduced | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Method of introduction | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Initial concentration of byproduct material in the product or material | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 5. Maximum concentration of the radioisotopes in the product or
material at the time of transfer to persons exempt from licensing |
_______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 6. Control methods to assure that no more than the specified maximum concentration is in the product at the time of transfer | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 7. Estimated time interval between introduction and transfer of
the product or material |
_______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | Provides reasonable assurance of the following: | _______ |
| 1. Concentrations of byproduct material at time of transfer will not exceed the concentrations in � 30.70 | ||
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Reconcentration of the byproduct material in concentrations exceeding
those specified in � 30.70 is not likely |
_______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Use of lower concentrations is not feasible | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. the product or material is not likely to be incorporated in any food, beverage, cosmetic, drug or other commodity or product designed for ingestion or inhalation by, or application to, a human being | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Applicants must submit information on Quality Assurance (QA) Program, including: | _______ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Describe radiation detection equipment and shielding used to identify and quantify the radioactivity induced in the product or material | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Specify the frequency, standards, and procedures used to calibrate such radioactivity detection equipment | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Describe counting procedures and how external measurements are converted to concentration values in terms of microcuries per gram | _______ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ | |
| Termination | ________ |
I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| A. | Applicant satisfied general requirements in 30.33 | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Applicant submits sufficient information regarding product pertinent to evaluation of potential radiation exposure, including: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Chemical and physical form and maximum quantity of BPM in each product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Details of construction and design of product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Method of containment or binding of BPM in product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Procedures for prototype testing to demonstrate that the material will not become detached from the product or that BPM will not be released under severe conditions | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 5. Results of prototype testing | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 6. Quality control procedures to be followed in the fabrication, and the quality control standards the product will be required to meet (�.15) | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 7. Proposed method of labeling or marking each unit, except for timepieces or hands or dials containing H-3 or PM-147 and its container with the identification of the manufacturer or initial transferor and the BPM | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 8. For products with limits specified in �.15, the radiation level and method of measurement | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 9. Any additional information, studies, and tests regarding product safety | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | Each product will contain no more than the quantity of BPM specified for that product in �.15 | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
�.14d(1): Timepieces |
||
| The byproduct material is properly contained in the product under the most severe conditions that are likely to be encountered in normal use and handling when hands, dials and pointers are subjected to the following prototype tests (the type or manner of tests(s) used will be dependent upon the form of the byproduct material): | ||
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| �.14: Certain Items | ||
| 1. Vibration tests | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Bending of hands or pointers over cylinder | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Immersion tests | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Other tests | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
�.40: Schedule A-Lock Illuminators |
||
| Prototype testing for automobile lock illuminators must consist of the following tests: | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. 100 hours of accelerated weathering | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Dropped onto concrete or iron from 3 feet 100 times | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Vibration tests | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Immersion in 30 inches of water for 24 hours to include a pressure (bubble) test | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 5. After each test, prototypes must be examined for evidence of physical damage | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ | |
| Termination | ________ |
I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| A. | Applicant satisfied general requirements in �.33 | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Product is designed to be used only for sand-consolidation in oil wells | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | Applicant must submit the following information: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. General description of product to be manufactured or transferred | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Description of control procedures for concentrations not to exceed 1.4 X 10-3 礐i/ml of final product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Each container of such product must contain durable, legible label with the following information: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Product name | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. State that product contains Scandium-46 and is designed only for sand-consolidation in oil wells | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Instruction for proper use | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Manufacturer's name | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. | Handling |
| Although the quantities of radioactive material contained in these products is extremely small, the basic radiation principles of time, distance, and shielding should be practiced as effective methods for minimizing exposure. | |
| Use of radioactive material should be only by responsible persons in authorized areas. | |
| Eating, drinking, smoking, and the application of cosmetics should be prohibited in areas of use. | |
| Gloves and laboratory coats should be worn when working with liquid radioactive material. | |
| 2. | Use |
| Exempt quantity licensed products containing radioactive material should be used only as intended by the manufacturer and in accordance with the instructions provided with the products. | |
| 3. | Storage |
| All radioactive materials should be securely stored when not in use. | |
| 4. | Disposal |
| These exempt distribution products may be disposed of in regular waste without regard to their radioactive content providing the customer is not a specific licensee and all radiation symbols have been removed or defaced. If the customer (laboratory/academic institution) receiving the exempt quantity is a specific licensee, then the customer is subject to the requirements of 10 CFR Part 20 in areas where 10 CFR 30.18 is silent (e.g., waste disposal). |
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ | |
| Termination | ________ |
I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| A. | Applicant satisfies �.33 for the manufacture, distribution, and transfer of exempt quantities of BPM except for a license to transfer BPM manufactured, processed, produced, packaged, or repackaged pursuant to a license issued by an Agreement State | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | The BPM is not contained in any food, beverage, cosmetic, drug, or commodity designed for ingestion, inhalation by, or application to humans | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | The BPM is not incorporated into any manufactured or assembled commodity, product, or device intended for commercial distribution | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Applicant has submitted copies of prototype labels and brochures for approval | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
�.19: CONDITIONS FOR LICENSE UNDER �.18 |
||
| A. | No more than 10 exempt quantities set forth in �.71, Schedule B shall be sold or transferred in any single transaction (an individual exempt quantity may be composed of fractional parts so that the sum does not exceed unity) | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Each quantity shall be separately and individually packaged with no more than 10 individual packages contained in any outer package for transfer. The external surface dose rate of the outer package must not exceed 0.5 mrem per hour | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | The immediate container shall bear a durable, legible label which: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Identifies the radioisotope and quantity of activity | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Bears the words, "RADIOACTIVE MATERIAL" | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Label or accompanying brochure shall state: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Contents are exempt from NRC or Agreement State licensing requirements | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Bear the words, "RADIOACTIVE MATERIAL -- NOT FOR HUMAN USE INTRODUCTION INTO FOODS, BEVERAGES, COSMETICS, DRUGS, OR MEDICINALS, OR INTO PRODUCTS MANUFACTURED FOR COMMERCIAL DISTRIBUTION IS PROHIBITED -- EXEMPT QUANTITIES SHOULD NOT BE COMBINED" | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Set forth additional radiation safety precautions and instructions
for handling, use, storage, and disposal of radioactive material |
________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ | |
| Termination | ________ |
I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| A. | Applicant satisfied general requirements in �.33 for the manufacture, distribution, and transfer of capsules containing carbon-14 urea for "in vivo"diagnostic use for humans except for a license to transfer BPM manufactured, processed, produced, packaged, or repackaged pursuant to a license issued by an Agreement State: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Applicant meets requirements under �.72(a)(2) requiring that the applicant is either registered or licensed with the U.S. Food and Drug Administrations or with a state agency as a drug manufacturer; or licensed as a pharmacy by a State Board of Pharmacy; or operating as a nuclear pharmacy within a Federal medical institution. | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | Applicant provides evidence that each capsule contains 36 kBq (1 microcuries) carbon-14 (allowing for nominal variation that may occur during the manufacturing process). | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Applicant confirms that the carbon-14 urea: | ________ |
| 1. is not contained in any food, beverage, cosmetic, drug (except as described in this section) or other commodity designed for ingestion or inhalation by, or topical application to, a human being; and | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. is in the form of a capsule, identified as radioactive, and to be used for its radioactive properties, but is not incorporated into any manufactured or assembled commodity, product, or device intended for commercial distribution. | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| E. | Applicant submits copies of prototype labels and brochures for approval. | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
�.21A: SAME: CONDITIONS OF LICENSE |
||
| A. | The immediate container must bear a durable, legible label which: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Identifies the radioisotope, the physical and chemical form, the quantity of radioactivity of each capsule at a specific date: and | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Bears the words "Radioactive Material." | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Label or accompanying brochure shall state: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. State that the contents are exempt from NRC or Agreement State licensing requirements | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Bear the words "RADIOACTIVE MATERIAL, FOR "IN VIVO" DIAGNOSTIC USE ONLY. THIS MATERIAL IS NOT TO BE USED FOR RESEARCH INVOLVING HUMAN SUBJECTS AND MUST NOT BE INTRODUCED INTO FOODS, MEDICINALS, OR INTO PRODUCTS MANUFACTURED FOR COMMERCIAL DISTRIBUTION. THIS MATERIAL MAY BE DISPOSED OF IN ORDINARY TRASH." | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. | PURPOSE | ||
| To set minimum performance requirements for gunsights containing tritium gas in glass vials for the purpose of producing light. | |||
| 2. | APPLICABILITY | ||
| All tritium illuminated gunsights registered after the date of this document are required to adhere to the requirements of this document. | |||
| Furthermore, all tritium illuminated gunsights licensed after the date of this document, for manufacture or distribution, are required to adhere to the requirements of this document. | |||
| New gunsights which can be shown by engineering evaluation to meet these criteria, because of similarity in design and assembly to gunsights passing this criteria, need not be subject to the prototype testing described in this document. | |||
| 3. | REQUIREMENTS | ||
| An applicant for a license to manufacture, process, or distribute gunsights containing tritium gas in vials for the purpose of producing light shall submit the information required in 10 CFR 32.22. The labeling requirements and prototype testing procedures in this document are considered sufficient to meet the requirements described in 10 CFR 32.22(a)(2)(x) and 32.22(a)(2)(xi), respectively. | |||
| 3.1 | DESIGN | ||
| Each gunsight is required to be designed so that: | |||
| 3.1.1 | In normal use and disposal of a single gunsight, it is unlikely the external radiation dose in any one year, or the dose commitment resulting from the intake of radioactive material in any one year, to a suitable sample of the group of individuals expected to be most highly exposed to radiation or radioactive material from the gunsight will exceed the dose to the appropriate organ as specified in Column I of Table O.1. | ||
| 3.1.2 | In normal handling and storage of the quantities of gunsights likely to accumulate in one location during marketing, distribution, installation, and servicing of the gunsights, it is unlikely that the external radiation dose in any one year, or the dose commitment resulting from the intake of radioactive material in any one year, to a suitable sample of the group of individuals expected to be most highly exposed to radiation or radioactive material from the gunsight(s) will exceed the dose to the appropriate organ as specified in Column II of Table O.1. | ||
| 3.1.3 | It is unlikely that there will be a significant reduction in the effectiveness of the containment, shielding, or other safety features of the gunsight from wear and abuse likely to occur in normal handling and use of the gunsight during its useful life. | ||
| 3.1.4 | In use and disposal of a single gunsight, or in handling and storage of the quantities of gunsights likely to accumulate in one location during marketing, distribution, installation, and servicing of the gunsight, the probability is low that the containment, shielding, or other safety features of the gunsight would fail under such circumstances that a person would receive an external radiation dose or dose commitment in excess of the dose to the appropriate organ in Column III of Table O.1, and the probability is negligible that a person would receive an external radiation dose or dose commitment in excess of the dose to the appropriate organ in Column IV of Table O.1. | ||
| Part of Body | Col. I (rem) | Col. II (rem) | Col. III (rem) | Col. IV (rem) |
|---|---|---|---|---|
| Whole body; head and trunk; active blood-forming organs; gonads; or lens of eye | 0.001 | 0.01 | 0.5 | 15 |
| Hands and forearms; feet and ankles; localized areas of skin averaged over areas no larger than 1 square centimeter | 0.015 | 0.15 | 7.5 | 200 |
| Other organs | 0.003 | 0.03 | 1.5 | 50 |
| 3.1.5 | It is engraved with H-3 and the name, registered trademark, or license number of the manufacturer, processor, producer, or initial transferor of the gunsight. | ||
| The gunsight may be labeled using paint or a durable metal foil label. However, the applicant must provide adequate information that the labeling will remain legible after being subject to the prototype testing described in this document. | |||
| 3.2 | PROTOTYPE TESTING | ||
| At least five gunsights of each model are to be subject to each of the tests described below. The same gunsight(s) are to be used for each test. Order of the testing is not significant. Between each test the gunsights are to be visually inspected to ensure there have been no detrimental effects to the gunsights. The gunsights must not become loosened or detached from the guns (tests 3.2.7 & 3.2.8) and the light sources must not become loosened or detached from the gunsights as a result of any of the tests. Once all tests are completed, the gunsights are to be subject to the evaluation in section 3.2.9. | |||
| 3.2.1 | CHEMICAL | ||
| The gunsight is to be immersed for 48 hours at room temperature in each of the following: | |||
| gun oil | |||
| trichloromethane | |||
| cleaning compound according to MIL-C-372B | |||
| 3.2.2 | TEMPERATURE | ||
| High Temperature: The temperature of the gunsight is to be raised from ambient to 120C and held at this temperature for one hour. | |||
| Low Temperature: The temperature of the gunsight is to be lowered from ambient to -46C and held at this temperature for 48 hours. | |||
| Relative Humidity: The gunsight is to be placed in an environment of 100% relative humidity and a temperature of 42 C and held in this environment for 48 hours. | |||
| 3.2.3 | TEMPERATURE SHOCK | ||
| The gunsight is to be heated to 80C and held at this temperature for 15 minutes. The gunsight is to be transferred, within 15 seconds, to a cold chamber having a temperature of -46C and held in this chamber for 15 minutes. If water is used as the cold chamber, it is to be flowing at a rate of at least 10 times the gunsight volume per minute. If the water is stationary, the water volume is to be at least 20 times the volume of the gunsight. | |||
| 3.2.4 | VIBRATION | ||
| The gunsight is to be subject to simple harmonic motion having an amplitude of 0.075 cm. The vibration cycle is to go from 10 Hz to 50 Hz and back again in approximately 1 minute. This is to be carried out for 10 cycles. Afterwards, the gunsight is to be subject to 30 minutes of vibration at resonance frequency. | |||
| This test is to be carried out in each of the three principal axes of the gunsight. | |||
| 3.2.5 | PRESSURE | ||
| The gunsight is to be placed in a test chamber and exposed to 0.25 and 2.0 bars for 4 periods of 15 minutes each, the pressure being returned to atmosphere between each period. | |||
| 3.2.6 | PENETRATION | ||
| A hammer with a small point and weighing 10 g is to be dropped from a height of 1 meter onto the exposed surface of the light source. | |||
| 3.2.7 | MECHANICAL SHOCK | ||
| This test is to be performed with the gunsight attached to the gun which would have the most detrimental effect on the gunsight. | |||
| The gun is to be dropped from 2 meters onto a hard surface (at least 1" thick 85 durometer rubber backed by concrete). The gun is to be dropped 60 times in such a manner that it strikes the surface ten times in each of the following attitudes: | |||
| Barrel vertical, muzzle down | |||
| Barrel vertical, muzzle up | |||
| Barrel horizontal, bottom up | |||
| Barrel horizontal, bottom down | |||
| Barrel horizontal, left side up | |||
| Barrel horizontal, right side up. | |||
| 3.2.8 | FIRING | ||
| This test is to be performed with the gunsight attached to the gun which would have the most detrimental effect on the gunsight. The gun is to sequentially fire 5000 rounds of ammunition with lapses only sufficient to allow reloading (only one of each model site needs to be subjected to the firing test). | |||
| 3.2.9 | EVALUATION | ||
| After each test, the gunsight is to be immersed in water for 24 hours at ambient temperature. The volume of the water is to be about equal to 10 times that of the volume of the gunsight. After the gunsight is removed, the activity of the solution is to be measured. The activity of the solution is to be less than or equal 50 nanocuries. | |||
1. American National Standards Institute, Inc., ANSI/SAAMI Z299.5-1985 American National Standard Voluntary Industry Performance Standards Criteria of New Firearms Designs Under Conditions of Abusive Mishandling for the Use of Commercial Manufacturers (Connecticut: Sporting Arms and Ammunition Manufacturers' Institute, Inc., 1985)
2. American National Standards Institute, American National Standard N540; Classification of Radioactive Self-Luminous Light Sources (Washington: U.S. Government Printing Office, 1976)
3. Department of the Army, US Army Weapons Command, Supplementary Quality Assurance Provisions No. 12002965 Low Light Level Sight Kit; M16/M16A1 Rifle
4. DIN, Sealed Radioactive Sources, Requirements and Classifications, DIN No. 25426 (Germany)
5. Fabrique Nationale Herstal S.A., Branche Defense Et Securite Procurement Quality Specification No. 390.700.501/1 Sights, Night Use (Belgium)
6. International Atomic Energy Agency, Safety Series No. 23 Radiation Protection Standards for Radioluminous Timepieces (Vienna: International Atomic Energy Agency, 1967)
7. Ministry of Defense, Defense Standard 62-4/Issue 3 Lamps/Nuclear (Gaseous Tritium Light Sources) (London: Ministry of Defense Directorate of Standardization, 1976)
8. Regulations Relating to the Radioactivity of Watches and Clocks (Germany:1984)
| A. | Normal conditions | ||
| 1. | Normal use | ||
| No radiation dose commitment is anticipated during normal use of the gunsight systems. External radiation dose rate at 25 cm is estimated to be less than 0.001 mrem/hr. The tritium gas is sealed in borosilicate glass, therefore no inhalation or ingestion of the radioactive material is expected in normal use. | |||
| 2. | Storage | ||
| Distilled water immersion tests on the sights indicated a leakage rate no greater than 1 E-5 礐i/sight in 24 hours. Assuming that 8000 units containing three tritium sources each and 2000 units containing one source each are stored in a 14 ft x 10 ft room in a 65,000 sq ft warehouse with an air exchange rate of 1 air change per hour, the calculated equilibrium concentrated of tritium is as follows: | |||
| where: | |||
| I = rate of influx of H-3 gas | |||
| V = volume of the room | |||
C = equilibrium H-3 gas concentration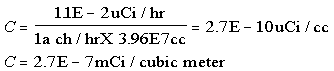 |
|||
| I = 26,000 sights x 1 E-5 礐i/sight - 24 hr = 1.l E-2 礐i/hr | |||
| V = 1400 cubic ft x 2.83 E4 cc/cubic foot = 3.96 E7 cc | |||
| The concentration limit set in 10 CFR Part 20, Appendix B, Table 2, Column 1 for H-3 in air is 2 E-7 礐i/ml. The calculated equilibrium concentration in the storage area is less than 1% of the 10 CFR 20 concentration limit for a controlled area. | |||
| The annual dose commitment to a warehouse worker, working in the area for 1 hour/day, 250 days/year is as follows: | |||
| Assume: | |||
| � All H-3 gas is converted to tritiated water | |||
| � Total rate of absorption of tritiated water into body fluids (mCi/minute) from inhalation and skin absorption is 3 E-2 C where C is the concentration of tritiated water in air in mCi/cubic meter (ICRP 30) | |||
| � Committed dose equivalent per unit intake of tritiated water is 1.7E - 11 Sv/Bq (6.3 E-2 rem/mCi) | |||
| � Annual committed dose: | |||
| H = 2.7 E-7 mCi/cubic meter x 3 E-2 mCi-cubic meter/mCi-minute x 60 minute/hour x 250 hr/yr x 6.3 E-2 rem/mCi = 7.7 E-6 rem/year | |||
| H = 0.008 mrem/year | |||
| A similar type calculation in NUREG/CR-0 |
|||
| 8.3E4 mrem-cc/礐i-hr x 250 hr/yr x 2.7 E-10 礐i/cc = 0.006 mrem | |||
| 3. | Transportation | ||
| Assume a truck driver transports all sights to be stored in the warehouse in a single truckload and spends a total of two hours in the trailer loading and unloading. | |||
| V = 2.9 E7 cc (NUREG/CR-0215) | |||
| I = 1.1 E - 2 礐i/hr | |||
| Dose commitment: | |||
| H = 3.8 E-7 mCi/cubic meter x 3 E-2 mCi-cubic meters/mCi-minute x 2 hours x 60 minutes/hour x 6.3E-2 rem/mCi | |||
| H = 8.6 E-8 rem = 8.6 E-5 mrem | |||
| 4. | All other situations during normal use, storage, and transportation involve smaller quantities of H-3 and/or shorter exposure times thus would result in negligible dose commitment. | ||
| 5. | Disposal | ||
| The gunsights are relatively expensive items and are unlikely to be inadvertently removed from the firearm and disposed of. The disposal of an intact firearm to normal trash is unlikely. Instructions accompanying the sights request return of damaged or defective sights to the distributor. Therefore, improper or careless disposal of the sights is unlikely to cause any significant radiation dose. | |||
| NUREG/CR-0215 estimates the dose commitment to the maximally exposed individual for burial of 500,000 tritium lighted wristwatches per year in landfills (20,000 in a single location) to be 0.1 mrem/yr. If the sources are burned a potential maximum dose commitment of 17 mrem/yr was estimated. | |||
| The total number of gunsights potentially disposed of in a single year would be much lower and the H-3 activity per unit also lower by a factor of seven than that postulated for watches containing H-3. Therefore, disposal of gunsights will not present a radiation hazard to the general public. | |||
| B. | Accident conditions | ||
| 1. | Use | ||
| The maximum credible accident involving the use of the gunsight system is rupture of the source and instantaneous release of the gas during firing. Only the rear sight is of consequence since it is much closer to the breathing zone of the user than the front sight. | |||
| Assume: | |||
| � Rear sight contains a total of 12 mCi of H-3 gas | |||
| � Rear sight is 15 cm from the user's face | |||
| � Breathing zone can be represented by a cone with apex at the source and base, a 10cm diameter circle at the user's face | |||
| � All H-3 is converted to tritiated water instantly | |||
| � Effective half-time for tritiated water = 10 days | |||
| � Total absorption of inhaled tritium in body fluids | |||
| � Mass of soft tissue = 63,000 g (ICRP 30) | |||
| Fraction of gas released in the direction of the breathing zone: | |||
| where | |||
| r = radius of the base of the cone | |||
| R = distance from source to nose | |||
 |
|||
| Maximum estimated dose commitment to user assuming all H-3 gas is converted to tritiated water | |||
| H = 12 mCi X 0.03 X 6.3 E - 2 rem/mCi = 23 mrem | |||
| For such an accidental instantaneous release, most of the gas would remain as elemental H-3. The dose commitment from H-3 gas would be approximately 1000 times less. The total estimated dose commitment would be 2% of the calculated value since up to 2% of the gas originally in the glass capsule could be in the form of tritiated water. | |||
| 2. | Storage | ||
| The maximum credible accident involving storage of the units would involve a fire in the storage area which ruptures some of the borosilicate glass capsules. (A massive fire which would rupture all sources would be likely to result in immediate dispersion of the H-3 gas and dilution with outside air, thus reducing the concentrations of H-3 gas in the storage area. | |||
| Assume: | |||
| � 50% of the sources ruptured | |||
| � Immediate dispersion of the gas within the storage area | |||
| � Conversion of all H-3 gas to tritiated water | |||
| Total rate of absorption of tritiated water into body fluids (mCi/minute) from inhalation and skin absorption is 3 E-2 C where C is the concentration of tritiated water in air in mCi/cubic meter (ICRP 30) | |||
 |
|||
| Dose commitment: | |||
| H = 3.3 E3 mCi/cubic meter x 3 E-2 mCi - cubic meter/mCi-min x 6.3 E-2 rem/mCi | |||
| H = 6.2 rem/minute | |||
| Dose commitment to fireman remaining in enclosed area without respiratory protection for 2 minutes for purpose of rescue = 12 rem | |||
| This calculation greatly overestimates the true dose commitments in this situation. Air currents would disperse the gas very rapidly in the case of a fire, particularly one of such severity as to rupture 50% of the sources instantaneously. In addition, only a small fraction of the H-3 gas is likely to be converted to tritiated water before venting to the outside. | |||
| A more reasonable estimate of the dose commitment would be obtained using the maximum fraction of tritiated water in the source, 0.02. If this value is used the dose commitments become 12 mrem for the occupant and 24 mrem for the fireman. | |||
| 3. | Ingestion or inhalation of the entire H-3 content of the front sight (17 mCi). | ||
| H = 17 mCi x 6.3 E-2 rem/mCi = 1.1 rem | |||
| The calculation assumes the entire 17 mCi H-3 gas is converted to tritiated water. H-3 gas is not absorbed readily in body fluids thus produces negligible dose. This postulated accident would require that an individual remove the source from the sight without damaging it, swallow it, and have the source rupture while in the digestive tract. Each of these conditions is highly improbable. The combination of all three occurring is nearly impossible. | |||
Compliance with 10 CFR 32.23 and 32.24 |
|||
| A. | Normal use and storage | ||
| No radiation dose commitment is expected in normal use of the gunsight system. The maximum expected dose commitment to workers in the storage area is less than 1 mrem/year. This is within the limit set in Column II, Table O.1. | |||
| B. | Accidental release of the tritium gas | ||
| 1. | Under maximum credible conditions of use of the equipment, the dose commitment to an individual would not exceed 23 mrem, within the limits set in Column III, Table O.1. In the highly improbable case where an individual ingested the contents of an entire source, the estimated dose commitment is 1 rem. This is within the limits set in Column IV, Table O.1. | ||
| 2. | Under extreme fire conditions in the storage area, the estimated maximum dose commitment to an occupant of the area is 6 rem; to a fireman in the process of rescue, 12 rem. More reasonable values based on 2% of the H-3 gas being oxidized and remaining in the storage room are 12 mrem and 24 mrem respectively. However, even under the extreme conditions the dose commitments would be within the values in Table O.1. | ||
Disposal of Units |
|||
| No significant radiation dose commitment is expected to result from disposal of the gunsights since rapid dispersion and dilution with the atmosphere would rapidly reduce tritium concentrations in air to background levels. | |||
| Users of the devices are instructed to return defective units and unwanted units to the distributor for disposal. The cost of this product is such that inadvertent or careless disposal is unlikely. | |||
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ |
I certify that I have reviewed the licensee's request dated ________ , as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| Mail Control No.: ________ | ||
�.22: SELF-LUMINOUS PRODUCTS (�.19) |
||
| Completed sealed source and device evaluation resulting in issuance of Registration certificate No. NR-XXX-X-XXX-E | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| To manufacture, process, produce, or initially transfer self-luminous products containing H-3, Kr-85, or Pm-147, the applicant must satisfy �.33(except in agreement States) and provide the following information: | ________ | |
| A. | Description of product and intended use | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Type and quantity of BPM per unit | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | Chemical and physical form of BPM and changes that may occur during the useful life of the product | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| D. | Solubility in water and body fluids of the forms in �.22(a)(2)(ii) | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| E. | Details of construction and design as related to containment and shielding and other safety features under normal and severe conditions of handling, storage, use, and disposal | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| F. | Maximum external radiation levels at 5 and 25cm from external surface of product an the method of measurement | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| G. | Degree of access to human beings during normal use | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| H. | Total quantity of BPM expected to be distributed annually | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| I. | Expected useful life of product | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| J. | Proposed method of labeling or marking each unit with: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Manufacturer or initial transferor of product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. BPM in product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| K. | Procedures for prototype testing (containment, shielding and other safety features) in both normal and severe conditions | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| L. | Results of prototype testing including any change in form, extent of release to environment, increase in radiation levels and changes in safety features | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| M. | Estimated external radiation doses and dose commitments | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| N. | A determination that the criteria of �.23(d) will be met | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| O. | Quality Control procedures followed in fabrication of production lots of product and Quality Control standards product must meet | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| P. | Any additional studies and tests | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| Mail Control No.: _______ | |
| Amendment No.: ______________ | Expiration Date: ______________ |
| License No.: ______________ | Program Code: ______________ |
| Docket No.: ______________ | Reference No.: ______________ |
| Licensee Name: ________________________________________________________ | |
| Address: _______________________________________________________________ | |
| ______________________________________________________________________ | |
| Action Type: | New License | ________ |
| New License/Licensee | ________ | |
| Renewal | ________ | |
| Product Transfer Report | ________ | |
| Amendment | ________ |
I certify that I have reviewed the licensee's request dated ________, as supplemented by any letters referenced in the license and in accordance with guidance provided by the Office of Nuclear Material Safety and Safeguards, appropriate Standard Review Plans and regulations, and the attached checklist.
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Reviewer | Date |
| ________________________________________ | ________ |
| Person Signing the License | Date |
GENERAL COMMENTS: __________________________________
________________________________________________________
________________________________________________________
| Mail Control No.: ________ | ||
�.26: GAS AND AEROSOL DETECTORS (�.20) |
||
| To manufacture, process, or produce gas and aerosol detectors containing BPM, and designed to protect from fire | ________ | |
| A. | Applicant must satisfy �.33 (except in Agreement States) | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Submit the following information: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Description of product and intended use | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Type and quantity of BPM per unit | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Chemical and physical form of BPM and changes that may occur during the useful life of the product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Solubility in water and body fluids of the forms in �.26(a)(2)(ii) | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 5. Details and design as related to containment and shielding and other safety features under normal and severe conditions of handling, storage, use, and disposal | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 6. Maximum external radiation levels at 5 and 25cm from external surface of product and the method of measurement | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 7. Degree of access to human beings during normal use | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 8. Total quantity of BPM expected to be distributed annually | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 9. Expected useful life of product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 10. Proposed method of labeling or marking (�.29(b)) | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 11. Procedures for prototype testing (containment, shielding and other safety features) | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 12. Results of prototype testing including any change in form, extent of release to environment, increase in radiation levels, and changes in safety features | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 13. Estimated external radiation doses and dose commitments | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 14. A determination that the criteria referred to in Ё32.27 and 32.28 will be met | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 15. Quality Control procedures followed in fabrication of production lots of product and Quality Control standards the product must meet | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 16. Any additional studies and tests | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
�.29(b): LABELS |
||
| A. | Each detector must contain a durable, legible, readily visible label or marking on external surface of detector containing: | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. "CONTAINS RADIOACTIVE MATERIAL" | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 2. Name and quantity of activity of BPM | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 3. Identification of the person licensed to transfer the product | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| B. | Label or marking is located where it will be readily visible when the detector is removed from its mounting | ________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| C. | The external surface of the point-of-sale package has a legible,
readily visible label or marking containing |
________ |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 1. Name and quantity of activity of BPM | ________ | |
| 2. Identification of the person licensed to transfer the product | ________ | |
| 3. The following or similar statement: | ________ | |
| THIS DETECTOR CONTAINS RADIOACTIVE MATERIAL AND HAS BEEN MANUFACTURED IN COMPLIANCE WITH U.S. NRC SAFETY CRITERIA IN 10 CFR 32.27. THE PURCHASER IS EXEMPT FROM ANY REGULATORY REQUIREMENTS | ||
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 4. Each detector and point-of-sale package is provided with any other information as may be required by the Commission | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
| 5. Applicant must maintain records and product transfer reports | ________ | |
| COMMENTS: _______________________________________________________________ | ||
| ___________________________________________________________________________ | ||
The following page shows an example of a Materials License in Letterhead Format.
MATERIALS LICENSE
[Licensee Name]
[Street Address/P.O. Box]
[City, State, Zip Code]
License No. X-XXXXX-XXE
Docket No. 030-XXXXX
Amendment No. XX
In accordance with application/letter dated ________ , NRC License No. XX-XXXXX-XXE is hereby issued/renewed in its entirety to read as follows:
Pursuant to the Atomic Energy Act of 1954, as amended; the Energy Reorganization Act of 1974, as amended (Public Law 93-438); 10 CFR Part 30, "Rules of General Applicability to Domestic Licensing of Byproduct Material"; Section 32.XX, 10 CFR Part 32, "Specific Domestic Licenses to Manufacture or Transfer Certain Items Containing Byproduct Material"; application dated ________ ; and letters dated ________ ; ________ ; and ________ ; a license is hereby issued to (Licensee's Name) to distribute (product such as calibration sources) containing (list radionuclides) in individual quantities not to exceed the amounts specified in Section 30.71, Schedule B, 10 CFR Part 30 (may list specific activities), to persons exempt from licensing pursuant to Section 30.18, 10 CFR Part 30, or equivalent provisions of the regulations of any Agreement State.
This license shall be deemed to contain the conditions specified in Section 183 of the Atomic Energy Act of 1954, as amended, and other applicable rules, regulations, and orders of the U.S. Nuclear Regulatory Commission, now or hereafter in effect, and to the following conditions:
1. This license does not authorize possession or use of licensed material.
2. The licensee is authorized to distribute only from its facility located at (locations or points-of-distribution).
3. The licensee shall submit periodic material transfer reports as specified in Section 32.XX, 10 CFR Part 32.
This license shall expire on [Expiration date].
| FOR THE U.S. NUCLEAR REGULATORY COMMISSION | |
| DATE: ________ | BY: ________________________ |
| [License Reviewer] | |
| Material Safety Branch | |
| Division of Industrial and Medical Nuclear Safety | |
| Office of Nuclear Material Safety and Safeguards | |
| Washington, DC 20555 |
[Date]
| License No. XX-XXXXX-XXE Docket No. 030-XXXXX Mail Control No. XXXXXX |
[Licensee's Name]
ATTN: [ ]
[Street/P.O. Box]
[City, State Zip]
SUBJECT: LICENSE RENEWAL APPLICATION
Dear [ ]:
This is to acknowledge receipt of your application for renewal of the materials license identified above. Your application is deemed timely filed and, accordingly, the license will not expire until final action has been taken by this office.
Any correspondence regarding the renewal application should reference your license number and the mail control number specified above.
Sincerely,
[Licensing Assistant]
|
Privacy Policy |
Site Disclaimer |