The American Urological Association will meet on April 25 - 30, 2009 in Chicago, Il, http://www.auanet.org/content/homepage/homepage.cfm  .
.
Meeting Poster (PDF, 235 KB) Printer friendly version of the contents on this page
NIDDK's Mission in Urology Research and Training
Overview
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) http://www2.niddk.nih.gov/ supports a broad range of basic and clinical research and training efforts relevant to benign urologic disease. The NIDDK's Division of Kidney, Urologic, and Hematologic Diseases (DKUH) houses the Urology Programs and has the primary responsibility for advancing the Institute's mission interests in urology.
- Urology Basic Science, including basic studies of the bladder, prostate, and the genitourinary tract
- Developmental Biology of the Urogenital Tract
- Urology Clinical Science and Clinical Trials
- Urology Women's Health Studies
- Urology Genetics and Genomics
- HIV/AIDS
- Pediatric Urology
- Urologic Diseases Epidemiology
- Urology Technology Development
The NIDDK promotes urology research and training through numerous activities, including:
- Funding of investigator initiated and Institute solicited individual research projects (e.g., R01s)
- Developing basic and clinical research networks
- Creating resources for investigators
- Enhancing training and career development
- Organizing scientific conferences and workshops
- Developing strategic plans to direct research efforts
- Advancing outreach efforts for the scientific and patient communities
- Promoting urology small business enterprises
- Collaborating with other Federal agencies, advocacy groups, professional organizations, etc.
The NIDDK Urologic Diseases Information Clearinghouse (NKUDIC) http://kidney.niddk.nih.gov/
The NKUDIC is an information dissemination service of the NIDDK. The NKUDIC was established in 1987 to increase knowledge and understanding of urologic and kidney disease among patients, their families, health care professionals, and the general public.
Urology Contacts
Telephone: (301) 594-7717
Genetics & Genomics Programs Rebekah Rasooly, Ph.D. rasoolyr@extra.niddk.nih.gov
Urology Programs Debuene Chang, M.D. changtd@mail.nih.gov
Epidemiology Program Paul W. Eggers, Ph.D. eggersp@extra.niddk.nih.gov
Development Program Deborah Hoshizaki, Ph.D. hoshizakid@niddk.nih.gov
Clinical Trials Programs John W. Kusek, Ph.D. kusekj@extra.niddk.nih.gov
Urology Training/Career Programs Tracy L. Rankin, Ph.D. rankint@mail.nih.gov
Urology Cell Biology Programs Chris V. Mullins, Ph.D. mullinsc@extra.niddk.nih.gov
Urology Programs Leroy M. Nyberg Jr., Ph.D., M.D. nybergl@extra.niddk.nih.gov
NIDDK Review Branch
The NIDDK Review Branch administers the review of applications responding to Institute specific solicitations and additional special application types.
NIDDK Review Branch Staff:
Review Branch Chief Francisco O. Calvo, Ph.D. calvof@extra.niddk.nih.gov
Review Branch Deputy Chief Michele Barnard, Ph.D. barnardm@extra.niddk.nih.gov
NIH Center for Scientific Review (CSR)
http://www.csr.nih.gov
The Digestive, Kidney, and Urological Systems Review Group (DKUS IRG) contains the Urologic and Kidney Development and Genitourinary Diseases (UKGD) Study Section. The UKGD serves as the primary study section for review of benign urology clinical and basic research applications directed toward the CSR. The scientific focus of the UKGD includes the normal and abnormal development of kidney, urinary tract, and the male genital system; as well as cellular, physiologic, and pathophysiologic processes of the bladder, prostate, genitourinary tract, and the pelvic floor.
CSR Staff:
DKUS IRG Chief Mushtaq Khan, Ph.D.khanmu@csr.nih.gov
UKGD Scientific Review Officer Ryan Morris, Ph.D. morrisr@csr.nih.gov
Training and Career Development
Pre- and Post-Doctoral Training Ruth L. Kirschstein National Research Service Awards (NRSA )
http://www2.niddk.nih.gov/Funding/TrainingCareerDev/
Training & Career Development Timeline
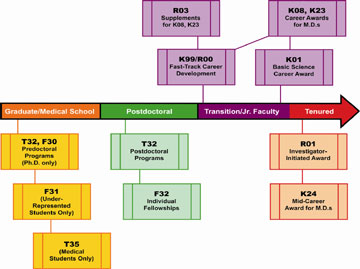
Loan Repayment Program
The purpose of the Extramural Loan Repayment Program is to ease the debt burden clinical scientists may have incurred while attending medical school and a residency program. The NIDDK has two loan repayment programs: one for clinicians and one for pediatricians. In addition to these NIDDK programs, the National Center on Minority Health and Health Disparities (NCMHD) sponsors two other loan repayment programs for clinicians: one for those involved in health disparities research and another for clinical researchers from disadvantaged backgrounds. Competitive applicants must demonstrate their commitment to a research career and have a debt-to-salary ratio of at least 20 percent. The Loan Repayment Program may repay up to a maximum of $35,000 a year toward each participant's outstanding eligible educational load debt, depending on total eligible repayable debt. For more details about eligibility and to apply online, visit http://www.lrp.nih.gov .
Career Development Awards
http://www2.niddk.nih.gov/Funding/TrainingCareerDev/
- K01 (Mentored Research Scientist Development Awards) Support Ph.D. scientists who have at least 3 to 5 years of postdoctoral training and who need to transition to independence.
- K08 (Mentored Clinical Scientist Development Awards) * Aimed at physician-scientists to transition them to independence.
- K23 (Mentored Patient-Oriented Research Career Development Awards) * Aimed at clinical investigators engaged in patient-based research.
- K24 (Investigator Awards in Patient-Oriented Research) Support mid-career physicians in patient-oriented research with funded clinical investigations and who are mentoring young clinicians.
- K25 (Mentored Quantitative Research Career Development Awards) Available to individuals with quantitative (e.g., engineering, mathematics, computer science, etc.) backgrounds who wish to pursue biomedical research.
K99/R00 NIH Pathways to Independence
The NIH has another opportunity for career development. This is an ideal award for talented postdoctoral candidates on the fast-track to a productive research career. Eligible applicants must have five-years or fewer of postdoctoral research experience and may not already have an independent faculty position. The first two years of the award, the K99 phase, are intended to be the mentored career-development phase. At the end of the second year, the applicant must have secured an independent tenure-track position to continue the final three years of the award as an R01. Unlike most career development awards, this opportunity does not require U.S. citizenship or permanent residency status, but the applicant must be able to remain in the U.S. to conduct the full five years of the proposed work. For additional information about this award, see http://grants1.nih.gov/grants/guide/pa-files/PA-09-036.html .
* NIDDK-funded K08 & K23 awardees may apply for a small grant (R03) to obtain additional funding during the last 2 years of their 5-year K award.
Note: All NIH fellowships and career development award mechanisms except the K99/R00 require U.S. citizenship or permanent resident status. Application forms and instructions are available via the NIH website. Completed applications must be submitted for specific deadlines to the NIH for evaluation by a panel of scientists. Once your application is reviewed, you will receive the written evaluation as well as a numerical “score,” which rates your application. The NIH Institutes fund the best applications submitted.
NIDDK Urology Research Highlights
Urinary Incontinence Treatment Network (UITN)
http://www.uitn.net/ 
The NIDDK sponsored UITN is a group of urologists and urogynecologists investigating treatments for urinary incontinence. The UITN is conducting a number of trials:
( T rial O f M id- U rethral S lings) - The TOMUS study will compare the outcomes of minimally invasive surgical procedures to treat stress urinary incontinence in women.
SISTEr ( S tress I ncontinence S urgical T reatment E fficacy T r ial) - This study compares the long-term outcomes of two common surgeries for stress urinary incontinence, the Burch and sling procedures. SISTEr has completed enrollment. Primary results are published (NEJM(2007)356(21):2143-55). The extended follow-up phase, E-SISTEr, is collecting data.
BE-DRI ( B ehavior E nhances D rug R eduction of I ncontinence) - This study will determine if the addition of behavioral treatment to drug therapy for the treatment of urge incontinence will make it possible to discontinue the drug and still maintain a reduced number of accidents. This trial is completed and primary results are pending.
NIDDK Prostate Research Strategic Plan
The NIDDK in collaboration with a committee of outside experts has developed a new strategic vision to guide future research efforts for basic science, epidemiology, translational science, and clinical studies of benign prostate disease. This Strategic Plan will guide future decisions by the NIDDK in developing new research efforts and will serve as a resource for the urology community in identifying high priority areas of investigation. For information on how to order the plan see: http://kidney.niddk.nih.gov/
*Recommended Presentations*
Findings relevant to current NIDDK-supported efforts will be reported at the Plenary Session of the 2009 AUA Meeting:
- Stromal-Epithelial Interactions in Prostate Development – David Rowley, PhD (Plenary Session, Sunday, April 26)
- Overlap Between Prostatitis and Other Pelvic Pain Syndromes – Kristene E. Whitmore, MD (Plenary Session, Monday, April 27)
- Re-Look at the Use of Amitriptyline – Phil Hanno, MD (Plenary Session, Monday, April 27)
- Impact of Fluid Mgt. on Fluid Intake and UI in the BE-DRI Trial for OAB – Elizabeth Mueller, MD (POD38, Tuesday, April 28)
MAPP Research Network
The NIDDK has established the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network in order to address the fundamental, underlying etiology and natural history of urologic chronic pelvic pain syndromes (UCPPS), including Interstitial Cystitis/Painful Bladder Syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Scientific areas of emphasis include: Patient Clinical Phenotyping, Epidemiology, Neurobiology, and Basic Science. Associations of UCPPS with potential co-morbid conditions is another major area of emphasis. See http://www.mappnetwork.org/  for more information.
for more information.
NIH Roadmap
http://nihroadmap.nih.gov/
Overview
The NIH, with input from a wide range of relevant communities, formulated the NIH Roadmap for Medical Research. The NIH Roadmap is designed to address the most pressing problems facing medical research. The NIH Roadmap identifies the most compelling opportunities in three main areas:
- New Pathways to Discovery –Invests in emerging and needed areas of research such as biological pathways and networks, structural biology, molecular libraries and imaging,
nanotechnology, bioinformatics, and computational biology.
- Research Teams of the Future –Supports both individual creativity and collaborative team efforts by supporting interdisciplinary research, high-risk research, and public-private
partnerships.
- Re-Engineering the Clinical Research Enterprise –Assists clinical research through
harmonizing regulatory policies, multidisciplinary training, development of new networking and diagnostic tools, and facilitating the establishment of academichomes for clinical and translational research.
These efforts are promoted in large part through published NIH Roadmap funding initiatives. Selected NIH Roadmap Funding opportunities of particular relevance to urology include:
For a complete list, see: http://nihroadmap.nih.gov/grants/index.asp
NIDDK Biorepository
http://www.NIDDKrepository.org 
The NIDDK Central Repositories store samples and data from large NIDDK-funded clinical studies. Materials/data are made available to the research community at the end of the study or when an interim phase is completed. There are 3 Central Repositories:
- Biosample Repository – Stores many types of biosamples
- Genetics Repository – Receives bio-samples to isolate DNA, etc.
- Data Repository – Maintains study databases
Sample and/or data are currently available from various studies, including:
- Interstitial Cystitis Clinical Treatment Group (ICCTG)
- Medical Therapy of Prostatic Symptoms (MTOPS)
- Boston Area Community Health (BACH) Survey
- Urinary Incontinence Treatment Network – SISTR (UITN)
- Interstitial Cystitis Database Study (ICDB)
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC)
NIH News!
NIH and the American Recovery and Reinvestment Act (ARRA)
The recent ARRA legislation provides an unprecedented level of funding to the NIH to help stimulate the U.S. economy through the support and advancement of scientific research. Numerous opportunities will be available to researchers over the next two years – including funding of new research projects and administrative assistance to ongoing projects. All investigators are encouraged to check the NIH and NIDDK websites for updates!
http://www.nih.gov/recovery/index.htm
http://www2.niddk.nih.gov/Recovery/
Multiple PI Recognition
NIH is currently implementing a policy to recognize multiple PIs on a single R01 grant (http://grants1.nih.gov/grants/multi_pi/index.htm).
Enhancing Peer Review at NIH
The NIH is currently working with the scientific community to identify ways to enhance the peer review system, including changes to review guidelines,
reviewers, and the length of the R01 grant application. Numerous important changes are being implemented. For updates see http://enhancing-peer- review.nih.gov/.
Grant Basics
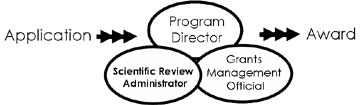
What's happening to my application?
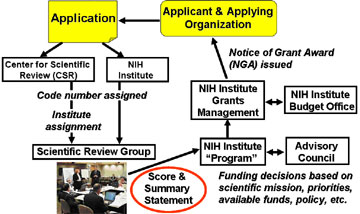
Which type of grant is best for me…?
R01 – Investigator Research Project (5 yrs; > $250K/yr)
R21 – Exploratory/Development Grants ($275K over 2 yrs)
F and T – Fellowship and Training Awards (varied)
K – Career Awards (varied)
R41/R42 – Small Business Technology Transfer (STTR) Program
R43/R44 – Small Business Innovation Research (SBIR) Program
What about a “Funding Initiative”?

Identify/Contact appropriate NIH staff
New PIs
http://www2.niddk.nih.gov/Funding/Grants/Resources_NewInvestigators.htm
The NIDDK has a strong commitment to the training and research funding of new investigators. Both the NIH and NIDDK have resources to assist new investigators, including:
- Peer-Review
All NIH peer reviewers are instructed to focus more on a proposed approach than a track record for new Principal Investigators (PIs).
- Second-Level Review
Automatic 2% boost in payline for a full five years of support! In
addition, all new-investigator R01 applications that receive a score in initial review receive special consideration by NIDDK staff. In FY2008, DKUH funded 30 new investigator R01s (part of the NIH-wide target of 1500 new PIs).
- New Director’s Bridge Award
All renewals for an investigator’s R01 are eligible for nomination for a New Director’s Bridge Award. This award is designed to provide
support while preparing to revise and resubmit.
- NIH High Priority, Short-Term Project Award (R56)
During second-level review, new investigators are given special
consideration for a small R56 award, which provides modest support for the PI to collect more preliminary data and submit an improved application.
- Career Development (K) awards, Small grants (R03) awards
and Mentoring Workshops .
Workshops
2009 Advancing Urologic Research – July 14–15, 2009. Bethesda, MD.
Genetics of GU Tract Malformations – Fall 2009. Washington DC area.
Stem Cells in Repair, Regeneration and Tissue Engineering –Winter
2010. Washington DC area.
Small Business
- Over $1 billion are available across NIH
- They provide seed money for high-risk projects
- They promote and foster partnerships with collaborators -including academia.
- Intellectual property rights are normally retained by small business
- Funds are NOT A LOAN -no repayment!
- Large corporations look to small companies for initial development
Small Business Innovation Research (SBIR)
http://www.zyn.com/sbir 
http://grants.nih.gov/grants/oer.htm
- The SBIR program supports innovative research conducted by smallbusinesses to develop products for commercialization. The PI must be
employed by the small business, but a research institution may be involved.
Small Business Technology Transfer (STTR)
http://www.zyn.com/sbir 
http://grants.nih.gov/grants/oer.htm
The STTR program supports innovative research for products that have the potential for commercialization. STTR projects must be conductedcooperatively by a small business and a research institution.