Introduction
How to Find Clinical Trials Using the Search Form
Help With Your Clinical Trial Search Results
Help with Understanding Trial Descriptions
How to Get Help from an Information Specialist
Introduction
The National Cancer Institute's (NCI's) PDQ® Cancer Clinical Trials Registry contains more than 8,000 active clinical trials (accepting participants) and more than 19,000 closed trials. These pages explain how to search for trials and review the results of your searches.
It is helpful to gather as much information as possible before a search. Information such as the specific type and stage of cancer, the type of trial that might be relevant (treatment, diagnostic, supportive care), and other details about the patient will be helpful. Speak with a health care provider to gather this information and make sure you review the information that you find with the health care provider.
Important Points
- In general, the more criteria you specify, the fewer clinical trials your search will retrieve. For a simple search, you can choose a Cancer Type, Stage/Subtype of Cancer, Location of Trial, and/or Type of Trial.
- To focus your search, you can specify more detailed information about the trials you wish to view or even search by a clinical trial identification number. The following pages describe each search option on the form, the various ways that you can display and sort your search results, and the information shown for each clinical trial in your search results.
- Skip any items on the form that you don't know or that don't apply to you. The default value for every search option is "All."
- Some features on this search form use JavaScript. If it is turned off in your computer's browser, these features will not work. The search options that use JavaScript are:
- Hospital/Institution selection
- Drug selection
- Treatment/Intervention selection
- Trial Investigator selection
- Lead Organization selection
 Message indicating JavaScript is turned off.
Message indicating JavaScript is turned off.
If you have questions while working with the search form, you can call 1-800-4-CANCER for help from an NCI cancer information specialist. You can also contact LiveHelp, NCI's online chat service, for help. The last section of this Help document has more details about LiveHelp and our phone service.
Back to Top
How to Find Clinical Trials Using the Search Form
The clinical trials search form allows you to search for trials using one or more criteria. Not all of the criteria are visible in the default display of the form. Some of the criteria are organized in groups, for example, Trial/Treatment Type includes Trial Type, Drug, and Treatment/Interventions. You can click on "Show Search Options" to see all of the options within a group.
 Default search view.
Default search view.
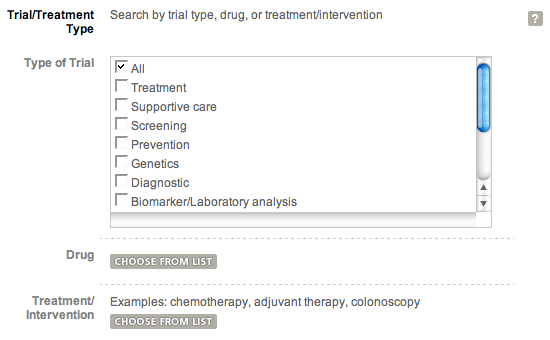 Expanded search option view.
Expanded search option view.
Each option on the form is described in the following sections with tips to make your search more effective. You can skip any options that you don't know or that don't apply to your situation.
Cancer TypeUse this list to choose the type of cancer being studied in a clinical trial(s), e.g., bladder cancer, prostate cancer. You also have the option of choosing "All." - If you want trials for any type of solid tumor, select "Solid tumor, unspecified."
- For trials that treat metastatic cancer, select "Metastatic cancer."
- You can also search for trials on cancer-related conditions by choosing either the specific condition (e.g., fatigue) or "Cancer- and cancer treatment-related conditions."
Stage/Subtype of Cancer After you choose a type of cancer, this list will show you the stages/subtypes for the selected cancer. The stage of cancer is the extent of cancer within the body. You may select one or more stages/subtypes or "All." - If you want a definition of the stage/subtype of cancer, please check the NCI Dictionary of Cancer Terms.
- If you choose "Cancer- and cancer treatment-related conditions" from the Cancer Type list, the Stage/Subtype box will show you a list of conditions that may result from having cancer or being treated for cancer.
Location of Trial
Use these options to search for trials near a specific ZIP Code, in a city, state, and country, at a Hospital/Institution, or at the National Institutes of Health (NIH) in Bethesda, Maryland. This search option works only for trials that are active (recruiting patients). Near ZIP Code Enter either your ZIP Code to search for trials in your area or the ZIP Code of another area of interest. - By default, searches are limited to within 100 miles of the ZIP Code that you enter. Use the drop-down list to change this to choices between 20 and 500 miles.
- The link to "ZIP Code Lookup" pops up a page from the U.S. Postal Service Web site. Find the ZIP Code by entering the address and clicking Submit. You can copy the ZIP Code from the pop-up window into the search form.
In City/State/CountryEnter a combination of city, state, and country to search for trials in that area. - You can select one or more U.S. states using the State list.
- The Country list includes non-U.S. states/provinces, such as Canada - Quebec.
At Hospital/InstitutionUse this search option if you are looking for trials conducted at a specific hospital or institution. Use the "choose from list" link to look up a hospital or institution name.
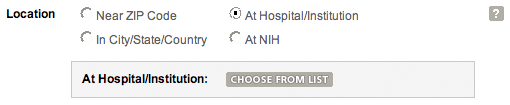 Location search by hospital/institution.
Location search by hospital/institution.
- When you click on "choose from list," a new window will pop up. You can search for a particular name or look through the list alphabetically.
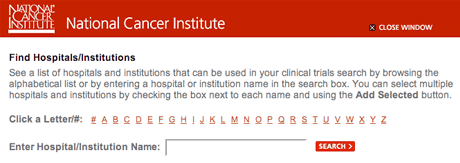 Hospital/institution selection window.
Hospital/institution selection window.
- Check the box next to the hospital(s) or institution(s) you want and click the "Add Selected" button at the bottom left of the pop-up window. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the names you selected.
- Use this field to find trials taking place at military or VA hospitals. To find VA facilities, use the "choose from list" and select the letter "V". This will display the VA facilities in the database.
At NIH OnlySelect the At NIH button to show only trials being conducted at the NIH Clinical Center (Bethesda, MD). See the NCI Fact Sheet on Cancer Clinical Trials at the NIH Clinical Center for more information.
Trial/Treatment TypeUse these options to search for a specific type of trial, or trials using a specific drug or intervention.
Type of TrialUse this list to choose the type of trial. Some trials fall into more than one category. You may check more than one box or select "All." The following trial types are available: - Treatment - trials that test how safe new treatments are and how well they work.
- Supportive care - trials that study ways to prevent or relieve side effects of cancer and its treatment, and ways to improve patients' quality of life during and after treatment.
- Screening - trials that check for cancer in people who do not have any signs of cancer.
- Prevention - trials that study ways to prevent cancer.
- Genetics - trials that study the genetic factors that may affect a person's risk of cancer or how a person will respond to cancer treatment.
- Diagnostic - trials that study tests and procedures to diagnose cancer, find out if cancer has spread in the body, or test how well cancer responds to treatment.
- Biomarker/Laboratory analysis - trials that study new biomarkers for cancer and changes that occur in cancer cells.
- Tissue collection/Repository - trials that collect cells or tissues from cancer patients for use in laboratory studies now or in the future.
- Educational/Counseling/Training - trials that study how patient counseling or training and education for patients and health care providers affect decisions about cancer prevention and treatment, or quality of life.
- Behavioral - trials that study how to help people behave in healthy ways to prevent cancer or detect it early.
- Natural history/Epidemiology - trials that study the long-term effects of cancer or its treatment in patients and the patterns, causes, and control of cancer in groups of people.
- Health services research - trials that study how health care is provided to people.
DrugUse the "choose from list" link to look up a drug name. - When you click on "choose from list," a new window will pop up. You can search for a particular drug or look through the list alphabetically. Check the box next to the drug(s) you want and click the "Add Selected" button at the bottom left of the pop-up window. The drug name(s) will be entered automatically into the form.
- The search results will list trials that include any of the drug names you selected unless you click the choice labeled, "Find trials that include all drugs shown."
- You can use different drug names such as, generic, brand/trade, and short names to search. For example, you can use Avastin (brand name) or bevacizumab (generic name).
Type of Treatment /InterventionUse the "choose from list" link to look up a treatment or intervention name. For example, use this option to find trials that are using "high-dose chemotherapy" or "brachytherapy". - When you click on "choose from list," a new window will pop up. You can search for a particular treatment or intervention or look through the list alphabetically. Check the box next to the treatment/intervention(s) you want and click the "Add Selected" button at the bottom left of the pop-up window. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the treatments/interventions you selected.
Keywords/Phrases Search for trials that contain the word or phrase you enter into the search box. For best results use this search option in combination with other search fields. For example, to search for breast cancer trials for a patient who is HER-2 negative, choose breast cancer from the Cancer Type option and enter "HER-2 negative" in the keyword box.
 Keyword search option.
Keyword search option.
- Use quote marks around a phrase to search for trials containing the whole phrase.
- Some trials you retrieve may contain the word or phrase as part of an exclusion criteria.
- Results are presented by order of relevance to the word or phrase entered.
- Using general words like cancer or disease will retrieve a large number of trials that may not be relevant.
Trial Status/Phase Use these options to search for trials by the status (active or closed) or by the phase of the trial, and to search for trials that were added within the last 30 days. Trial Status Use this option to choose whether to search for active trials (accepting new patients) or closed trials (no longer accepting new patients). - The default is for active trials.
- Closed trials may have published information describing what researchers have already studied and learned.
- Closed trials are not searchable by location.
Trial Phase Use this field to select the phase of trial. Most clinical trials are designated as phase I, II, III, or IV, based on the type of questions the trial is trying to answer. You may select one or more trial phases, or choose "All." - Phase I - trials that test the best way to give a new treatment (for example, by mouth, intravenous infusion, or injection) and the best dose.
- Phase II - trials that test whether a new treatment has an anticancer effect (for example, whether it shrinks a tumor or improves blood test results) and whether it works against certain types of cancer.
- Phase III - trials that compare the results of people taking a new treatment with the results of people taking the standard treatment (for example, which groups have better survival rates or fewer side effects).
- Phase IV - trials that evaluate side effects that were not apparent in the phase III trial.
- Phase may not be applicable to all trials, e.g., Natural history/Epidemiology trials.
New Trials Checking this box limits search results to only those trials added in the last 30 days. - New trials are posted to the Web site daily, Monday through Friday.
Trial ID/Sponsor Use these options to search for trials by protocol ID, sponsor, investigators, lead organization/cooperative group, or special category. Protocol ID An identification (ID) number that makes it easy to find a specific trial. Use this field only if you know the ID number or partial ID for a specific trial. - Enter one or more protocol IDs, separating them with commas or semicolons.
- The search results will list trials that include any of the IDs you entered.
Sponsor of Trial Use this option if you wish to select a sponsor category. For NCI trials, sponsorship is assigned based on how the trial is reviewed. For all others, assignment is based on who is coordinating or funding the trial. You may select one or more sponsors, or choose "All." - You can limit your results to trials sponsored by 1) one of the institutes or centers at the National Institutes of Health (NIH) on the list, 2) pharmaceutical companies, or 3) other medical/research institutions.
- Trials sponsored by one of the institutes or centers of the NIH can be located anywhere (not just the NIH campus).
Trial Investigators Use this option to select medical professionals and researchers who are conducting the trial. Use the "choose from list" link to look up an investigator name. - If you click on "choose from list," a new window will pop up. You can search for a particular name or look through the list alphabetically. Check the box next to the investigator(s) you want and click the "Add Selected" button at the bottom left of the pop-up window. The investigator name(s) will be entered automatically into the form.
- The search results will list trials that include any of the investigator names you selected.
Lead Organization or Cooperative Group Use this option if you wish to select an academic hospital, research institute, pharmaceutical company, cancer center, or cooperative group responsible for coordinating the trial. Use the "choose from list" link to look up the name. - Cooperative groups are NCI-funded groups of institutions, researchers, and community physicians. More information is available in NCI's Clinical Trials Cooperative Group Program.
- If you click on "choose from list," a new window will pop up. You can search for a particular name or acronym or look through the list alphabetically. Check the box next to the organization(s) or group(s) you want and click the "Add Selected" button at the bottom left of the pop-up window. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the names you selected.
Special Category Use this list to select a special trial category. You may choose more than one, or select "All." The following special categories are available: - CTSU trials - The Cancer Trials Support Unit (CTSU) is a project sponsored by NCI's Cancer Therapy Evaluation Program (CTEP) designed to give physicians and patients greater access to NCI-sponsored, phase III, adult cooperative group clinical trials. Cooperative Group sites located within the United States and Canada are currently eligible for participation in the CTSU. In addition, the CTSU is open to physicians and institutions in the United States who are not affiliated with a Cooperative Group.
- Group C-Treatment IND trials - These trials use Group C-designated drugs, investigational drugs found to have anti-tumor activity which are awaiting final FDA approval and are not yet available on the market.
- NCI-CMS pilot project trials - These trials were selected for a pilot project between the National Cancer Institute (NCI) and the Center for Medicare and Medicaid Services (CMS). Under this pilot project, the CMS will cover the routine and non-routine costs for these trials.
- NCI Avon award trials - Breast cancer trials funded by the NCI-Avon Progress of Patients Awards program, a partnership between NCI and the Avon Foundation, the charitable arm of Avon Products, Inc.
- NCI Cancer Bulletin featured trials - NCI-sponsored trials that were highlighted in a Cancer Bulletin article to give them added attention.
- NIH Clinical Center trials - Trials being conducted at the NIH Clinical Center in Bethesda, MD.
- SPORE trials - Trials funded through NCI's Specialized Programs Of Research Excellence (SPOREs). The goal of the SPORE program is to bring to clinical care settings novel ideas that have the potential to reduce cancer incidence and mortality, improve survival, and improve the quality of life. Laboratory and clinical scientists work collaboratively to plan, design, and implement research programs that impact on cancer prevention, detection, diagnosis, treatment, and control.
- Treatment Referral Center (TRC) trials - These trials offer investigational treatments for certain high priority agents or diseases and are available only at NCI-designated cancer centers.
Back to Top
Help With Your Clinical Trial Search Results
The Search Results page displays a list of trials that match the criteria that you selected on the search form. On this page, you can: - Click on the title of each trial to see a full description, including contact information.
- Read the trial titles and key information about the trials, as well as select the information you want to see for each trial.
- Sort the results in various ways and change the number of results you see on each page.
- Use check boxes to select the trials you want to print in the format you've chosen.
Choosing Display Options
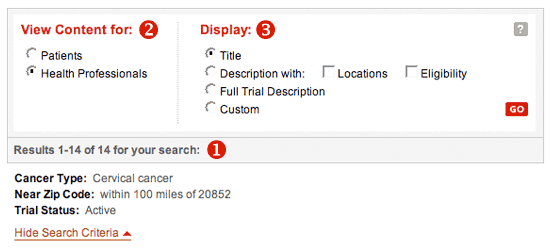 Display options for search results. Display options for search results.
- The number of trials that match your criteria and the number of trials displayed per page are shown. You can also see the search criteria that you entered in the search form or choose to hide the criteria.
- Use the "View Content For" buttons to choose to see information written for patients and the general public or for health professionals. The health professional trial descriptions have more detailed information and use words that are more technical.
- Use the "Display" buttons to select the information you want to see about the trial(s); then click "Go". The search results page will refresh and show the information in the format that you selected.
- Choose the "Description" button to see information about the purpose of the trial and other limited details. In addition, you can choose to see this description with the eligibility criteria (the requirements that must be met for an individual to be included in a trial). You can also choose to see the locations for the trial. The information shown in the description differs depending on your choice to see content for patients or health professionals.
- Choose the "Custom" button to create a custom display using information from the "Health Professional" trial descriptions written for NCI's PDQ clinical trials registry. This format is not available for the "Patient" version of trial descriptions or for descriptions imported from the National Library of Medicine's ClinicalTrials.gov database..
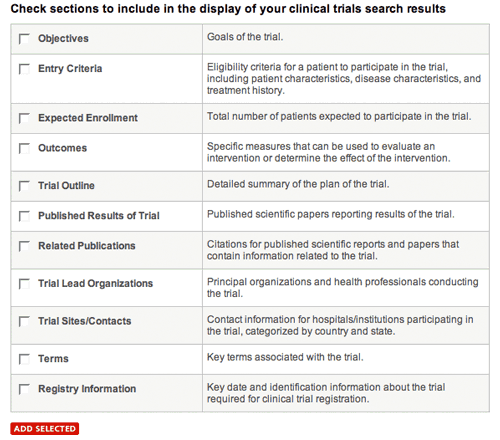 Options to customize display of search results.
Options to customize display of search results.
Use the check boxes to select the information you want to display for each trial. More Options for Displaying Search Results You can make changes to the order in which trials are displayed and the number of results shown per page. Remember to click the "Go" button to change your display.
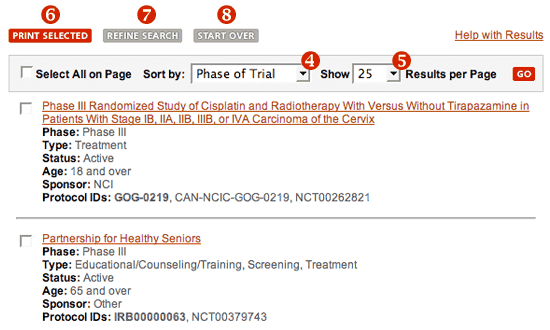 Other options for search results display.
Other options for search results display.
- Use the "Sort by" drop-down list to reorder the trials by one of the options listed. By default, the trials are ordered in descending order by phase:
- Phase IV
- Phase III
- Phase II
- Phase I
- No phase specified
If you enter keywords as part of your search criteria, the results will be ordered by relevance to the text you entered. - Use the "Show Results per Page" drop-down list to change the number of trials displayed on each page.
- Print: To print your search results, use the check boxes at the start or end of each trial description to select one or more trials for printing. Then click on the "Print Selected" button above the list of trials or at the bottom of the page.
- Refine Search: Your search results may include trials that are not exactly what you want, or you may wish to change some of your search criteria. The "Refine Search" button above the list of results and at the bottom of the page takes you back to the search form with your original criteria still in place. You may select additional search criteria or change already selected criteria.
- Start Over: To start a new search, click on the "Start Over" button above the list of results or at the bottom of the page.
Back to Top
Help with Understanding Trial Descriptions
This section will help you understand the information that is shown for each trial. Trial descriptions differ based on the source of the trial. Some trials are directly submitted to PDQ and others are imported from the National Library of Medicine's ClincalTrials.gov database. Details about trial descriptions are given below.
Description for Trials Submitted to PDQ
Title - The title of the trial gives key information about the trial. If you choose to view content for patients, the language of the title will be less technical.
Alternate Title - The alternate title is available for some trials. If you are viewing content for patients, the alternate title will be in more technical language. If you are viewing content for health professionals, the alternate title will be in less technical language.
Basic information about the trial is listed below the title. In some formats, it appears in a summary box.
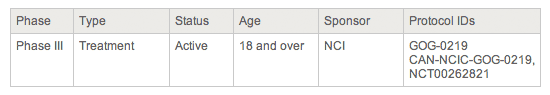 Basic information box in a trial description.
Basic information box in a trial description.
Phase - Gives information about how far along the drug/treatment/or procedure is in the testing process. The higher the Phase number, the more testing has been done. For definitions of each phase, see the section, How to Find Clinical Trials Using the Search Form. Type - Describes the overall purpose(s) of the trial. Sometimes multiple Trial Types are listed. See definitions of trial types, in the section How to Find Clinical Trials Using the Search Form. Status - Shows the current status of the trial. If you searched for Active trials (the default search), you will see one of the following statuses: - Active - The trial is currently accepting new patients.
- Approved, not yet active - The trial is not yet accepting new patients. It has received scientific approval, but may be waiting for some administrative steps to be complete.
If you selected "Closed" in the status field of the search form, you will see one of the following values: - Closed - The trial is not accepting new patients. Patients already on the trial may continue to be treated and/or followed.
- Completed - All aspects of the trial are complete. Patients are no longer being followed for data collection.
- Temporarily Closed - The trial is not accepting patients temporarily. Reasons for this may be short supply of the drug, safety issues with the trial, or administrative issues.
Age - Shows the age range of eligible participants for the trial. Sponsor - Shows who is sponsoring the trial. The National Cancer Institute or other NIH Institutes are identified by name. The other values in this column can be "Pharmaceutical/Industry" or "Other." Protocol ID - Shows one or more identification number(s) for the trial. One of the numbers displayed in this box may be preceded by the letters "NCT." This is the National Clinical Trial identifier provided by the National Library of Medicine's ClinicalTrials.gov database.
Patient Version of Trial Description
If you choose to view the information written for patients, the display includes the following : Trial Description - Each trial description contains the following sections: - Purpose - The type of trial being conducted, what treatments will be given or tests will be performed, and what researchers hope to accomplish.
- Eligibility - The requirements that a person must meet to participate in the trial. These requirements help researchers ensure the safety of participants and the scientific validity of the trial. For example, treatment trials may list type and stage of cancer, age, previous treatment, other health conditions, and more. The patient version of the trial description lists only some of the eligibility criteria. You can use the link to the health professional version of the trial description to see a more complete list.
- Treatment/ Intervention - The type of treatment or intervention, and how often it will be given. For non-treatment trials, a description of what happens on the trial is provided.
- Trial Lead Organizations - A list of one or more academic hospitals, research institutions, pharmaceutical companies, cancer centers, or cooperative groups responsible for coordinating the trial.
- Trial Sites and Contacts - The people or organizations conducting the trial. You or your health care provider can use the contact information to find out more about the trial, including eligibility, the enrollment process, and other details. Site information is not displayed for closed trials.
Health Professional Version of Trial Description This version provides information about the goals of the trial, the entry criteria for the trial, and what happens on the trial. You or your health care provider can use the contact information to find out more about the trial. Each trial description contains the following sections: - Objectives- A detailed description of the trial's goals.
- Entry Criteria- A list of the requirements a person must meet to participate in the trial. These are organized in three categories:
- Expected Enrollment -The number of participants expected to be included in the trial.
- Outcomes - The measures that will be used to find out if the trial meets the goals. The measures can be categorized as Primary or Secondary.
- Outline - A description of what will happen to the participant while on the trial. For a treatment trial, it is a description of the treatment plan including the "arms" or groups that participants may be assigned to on the trial.
- Published Results - Reference citations to published scientific reports and papers reporting results of the trial. A link may also be included to the abstract of the publication in the National Library of Medicine's PUBMED database. These are often available for Closed trials.
- Related Publications - Reference citations to published scientific reports and papers that contain information related to the trial. Sometimes results of a trial may be used in a publication that summarizes results from various trials or a review of a particular approach to treatment.
- Related Protocols - Links to trials associated with this trial.
- Related Web sites - Links to Web pages that provide more information about the trial. For example, some trials at NCI's Center for Cancer Research in Bethesda may link to a trial Web site.
- Trial Contact Information - Information about contacts that you can call or e-mail about the trial. You or your health care provider may want to look for contacts that are close to your location.
- Trial Lead Organizations - A list of one or more academic hospitals, research institutions, pharmaceutical companies, cancer centers, or cooperative groups responsible for coordinating the trial.
- Trial Sites - The people or organizations conducting the trial. They can provide more information about the trial, including eligibility and the enrollment process.
Registry Information - Details related to the laws and requirements for specific types of clinical trials to be registered in the National Library of Medicine's ClinicalTrials.gov database. It also includes a link to the record in the ClinicalTrials.gov database.
Description for Trials Imported from ClinicalTrials.gov Summary - An outline of the purpose of the study and a general description of what is being done. Further Study Information - More details about the study, for example, specific information about the treatment plan. Eligibility Criteria - A list of the requirements a person must meet to participate in the trial. The criteria may be labeled "Inclusion" or "Exclusion". See definition of eligibility criteria. Trial Contact Information - Information about contacts that you can call or e-mail about this trial. You or your health care provider may want to look for contacts that are close to your location. - Trial Lead Organizations - A list of one or more academic hospitals, research institutions, pharmaceutical companies, cancer centers, or cooperative groups responsible for coordinating the trial.
- Trial Sites - The people or organizations conducting the trial. They can provide more information about the trial, including eligibility and the enrollment process. Sometimes there may be a central number for all sites and the name of the hospital/institution may not be provided.
Back to Top
How to Get Help from an Information Specialist
If you do not want to search for trials on your own, you can get help by telephone from an experienced information specialist or use the live, online help available through NCI's Cancer Information Service (CIS). Our information specialists will help you perform a search while talking to you, or they will take information about your needs, perform the search for you after you talk, and send you the results.
- To get help from a specialist, call NCI's Cancer Information Service (CIS) at 1-800-4-CANCER (1-800-422-6237) between 9:00 a.m. and 4:30 p.m. local time, Monday - Friday. The CIS specialist will ask about your specific situation and provide information relevant to your needs.
- Live, online help is available through the CIS LiveHelp instant messaging service Monday - Friday, 9:00 a.m. to 11:00 p.m. U.S. Eastern time. Find the LiveHelp link in the left sidebar of the clinical trials search pages and at the foot of the search form and clinical trials search results pages.
Back to Top
|