HIV Vaccines: Where We Are and Where We’re Headed
With more than 6,500 new infections daily, HIV has assumed the dubious distinction of being one of the most catastrophic pandemics to confront mankind. Although the search for an HIV vaccine remains among the highest public health priorities, the identification of a preventive HIV vaccine has thus far eluded all efforts of the biomedical research community, mainly because of the significant scientific obstacles presented by the virus.
HIV mutates rapidly, hides from the immune system, and targets and destroys the immune system cells that are successful in fighting and clearing most other viruses from the body. To develop an HIV vaccine, researchers will have to outmanuever nature, unlike the situation with other viral diseases such as measles and influenza, where scientists have been able to induce protective responses with vaccines by mimicking natural infection.
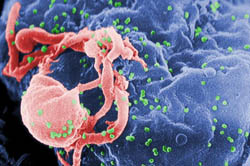 |
| Scanning electron micrograph of HIV emerging from a white blood cell. Green bumps on cell surface are sites of assembly and “budding” of virus particles. Credit: CDC/C. Goldsmith, P. Feorino, E. L. Palmer, W. R. McManus |
Challenges and Opportunities
NIAID and many other research organizations around the world conduct and support HIV vaccine research and, while advances have been made, there have been significant challenges. For example, in 2007 NIAID discontinued the STEP study, a large clinical trial in which a candidate vaccine failed to prevent HIV infection or reduce the amount of virus in those who became infected with HIV. More recently, NIAID decided that it will not conduct the HIV vaccine efficacy trial known as PAVE 100, which was to test a vaccine combination developed by the NIAID Vaccine Research Center (VRC) that included a product somewhat similar to the product tested in the STEP trial. Instead, NIAID announced it would consider a smaller, more focused clinical trial of the VRC vaccine combination.
Despite setbacks, the historic success of vaccines argues that HIV vaccine research must be continued, broadened, and accelerated. To those ends, NIAID is taking a more fundamental approach to invigorate and advance HIV vaccine discovery, shifting its focus to emphasize the importance of attracting new ideas to the field, developing better non-human primate (NHP) models to evaluate vaccine concepts, and designing NHP and clinical studies in ways to allow for more comparable results.
Attracting Innovative Ideas
NIAID has recently launched two research initiatives to stimulate new approaches for vaccine design: the Basic Vaccine Discovery program and the Highly Innovative Tactics to Interrupt Transmission of HIV program.
The Basic Vaccine Discovery program encourages innovative ideas from diverse scientific disciplines—including virology, immunology, and structural biology—to help overcome the obstacles presented by HIV. The program expects to support studies that do the following:
- Aim to better understand the induction of broadly neutralizing antibodies against HIV in order to identify vaccine designs that generate a broadly reactive immune response.
- Improve knowledge of the modulation of B- and T-cell responses leading to optimal generation of immunological memory by vaccines.
- Define correlates of protective immunity against HIV to guide researchers with future product design and testing.
The Highly Innovative Tactics to Interrupt Transmission of HIV program supports discovery, design, and early preclinical evaluation of highly innovative approaches to HIV prevention, including vaccines, that could provide long-term protection from acquiring HIV infection. The program seeks projects that explore novel hypotheses that address difficult problems, which if solved, could have a major impact on finding methods to interrupt HIV transmission. This initiative is open to new and established investigators and does not necessarily require research expertise in the area of HIV prevention.
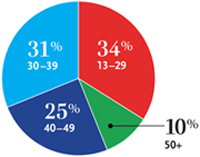 |
| This pie chart shows the estimated new HIV infections in 2006 by age. Credit: CDC. |
Expanding NHP Research
In addition, NIAID is expanding its research on NHP by partnering with other NIH institutes and external stakeholders to better track NHP research needs. These partnerships will ensure that the supply of primates keeps pace with research demands. Furthermore, NIAID’s HIV Vaccine Trials Network will promote collaboration between HIV vaccine scientists from NHP laboratories and clinical trial units, particularly new or young investigators, to strengthen the bridges between NHP and human research.
NIAID has also created a Vaccine Discovery Branch (VDB) within the Vaccine Research Program at the Division of AIDS to help accelerate the translation of basic lab discoveries into advances in HIV vaccine design. The VDB will plan, develop, implement, and evaluate grants and contracts to support the conduct of fundamental virology and immunology research related to novel vaccine concepts. Additionally, it will foster linkages and collaborations among investigators in different disciplines to broaden the scope of vaccine research and exploit findings in other fields.
The Search Continues
There are many promising avenues for preventing HIV infection, but none is more essential than a vaccine. Vaccines have eradicated some of history’s deadliest diseases and they are still the most efficient and cost-effective way to prevent illness, disability, and death from infectious microbes.
By concentrating resources on vaccine discovery in basic research laboratories, including those in NHP facilities and clinical research units, NIAID and it collaborators in government, academia, and industry aim to overcome the unique scientific challenges presented by HIV and help bring a safe and effective HIV vaccine closer to reality.
Related Links
back to top