RECORD SUMMARY OF THE REQUEST FOR INFORMATION ON OCCUPATIONAL EXPOSURE TO BLOODBORNE PATHOGENS DUE TO PERCUTANEOUS INJURY
EXECUTIVE SUMMARY
On September 9, 1998, the Occupational Safety and Health Administration (OSHA) published in the Federal Register a Request for Information (RFI) on engineering and work practice controls used to eliminate or minimize the risk of occupational exposure to bloodborne pathogens due to percutaneous injuries from contaminated sharps. OSHA has received 396 responses to the RFI. Comments were provided by more than 300 individual health care facilities, including nursing homes, clinics, and acute care, tertiary care, rehabilitation, and pediatric hospitals. Several organizations submitted combined responses on behalf of members representing over 130 additional healthcare facilities. Also responding were individual healthcare workers, researchers, unions, educational institutions, professional and industry associations, and manufacturers of medical devices. A review of the responses received permits several observations to be made:
- The OSHA 200 log, as it is currently being maintained, does not accurately reflect injuries that may involve exposure to bloodborne pathogens in healthcare facilities. The criteria established for recording occupational bloodborne pathogen exposures do not require recording of all injuries that pose a potential risk of disease transmission. Only those exposure incidents resulting in loss of consciousness, transfer to another job, restriction of work or motion, recommendation for medical treatment beyond first aid, or seroconversion are currently required to be recorded. Many facilities also apparently do not correctly interpret the established recording criteria.
- Those healthcare facilities that responded to the RFI have almost universally adopted surveillance systems in addition to the OSHA 200 log. These alternate systems commonly record all reported percutaneous injuries involving exposure to blood or other potentially infectious materials. Information is generally recorded on the circumstances under which injuries occurred, although this information appears to vary in content and level of detail.
- Although confounding factors exist, data submitted to the docket appear to indicate that the rate of percutaneous exposure incidents is declining. The best, most current, national estimate available in the docket is provided by the International Healthcare Worker Safety Center and is based on surveillance system data. This estimate indicates that approximately 590,000 percutaneous exposure incidents occur annually (Ex. 3-172V).
- Use of safer devices appears to be increasing in limited applications. Responses indicate that most IV line access is now accomplished using safer devices. However, safer devices appear to be used much less frequently in other applications.
- Responses indicate that safer medical devices are an effective and feasible method of hazard control in many instances. Nearly every healthcare facility responding to the RFI noted that a reduction in injuries had occurred after the introduction of a safer medical device.
- Training and education in the use of safer medical devices and safer work practices were repeatedly cited by respondents as effective means of preventing percutaneous exposure incidents. In addition, anecdotal responses indicate that staff involvement in the device selection and evaluation process can play an important role in achieving a reduction in injuries.
- Use of safer medical devices was generally not reported to have substantially affected the delivery of patient care.
- Increased costs and staff resistance to change were the most frequently reported obstacles to adopting safer medical devices. Other barriers encountered include equipment compatibility problems, facility purchasing agreement limitations, and the unavailability of effective safer medical devices for certain applications.
- Safer medical devices in general are more expensive than conventional devices. The total additional cost per facility, however, appears to be a small fraction of total healthcare costs, and reductions in the number of these injuries may result in substantial financial benefits from reduced costs for post-exposure testing and treatment as well as health benefits from a decrease in disease transmission.
- Although not generally identified as such, many respondents appear to consider a comprehensive safety and health program to be the most effective means of reducing the risk of bloodborne disease transmission. Information gathered through the surveillance system is commonly used for hazard identification and evaluation of program effectiveness; safer medical devices and safer work practices are used for hazard control; and respondents often regarded employee involvement along with effective education and training as essential.
- While healthcare facilities did not generally comment on an appropriate course of action for the Agency to take, a number of other respondents supported OSHA's interest in making safer medical devices available to employees. Some respondents, however, expressed reservations about any broad mandate requiring the use of safer medical devices, maintaining that the distinctive situations and needs of particular areas of practice (e.g., dentistry, anesthesiology) must be taken into account, and that device efficacy and effects on patient care should be established prior to device adoption.
TABLE OF CONTENTS
Introduction
Background
Responses to the Request for Information
Surveillance
Injury Rates
Recording of Injuries
Selection and Evaluation of Devices
Use of Safer Medical Devices
Training
Effectiveness of Safer Medical Devices in Reducing Injury Rates
Obstacles to Adoption of Safer Medical Devices
Cost Issues
Sharps Disposal Issues Page
Suggested Approaches to Reduce Infection Risk
Summary
Appendix A
Introduction
On September 9, 1998, the Occupational Safety and Health Administration (OSHA) published a Request for Information (RFI) in the Federal Register (63 FR 48250). The RFI requested information and comment on engineering and work practice controls used to eliminate or minimize the risk of occupational exposure to bloodborne pathogens due to percutaneous injuries from contaminated sharps. The following report is a summary of the information provided in the responses to the RFI. It summarizes the responses to the questions in the RFI and includes information provided to assist OSHA in identifying what approaches would effectively reduce the risk of disease transmission and what role the Agency may have in implementing these approaches.
The RFI has some limitations that must be recognized when analyzing the information provided. Respondents employ different methods of measuring injury rates and calculating the costs of injuries. These differences make comparison or aggregation of the data difficult. Also, no standard definition exists for the term "safer medical device." Many new devices have been developed to reduce the risk of needlesticks and other percutaneous injuries through elimination of the sharp or incorporation of safety features into the device. These devices can vary considerably in their clinical efficacy and effectiveness in reducing injury incidence. Consequently, respondents may have used differing interpretations of the term "safer medical device."
Notwithstanding these limitations, the responses to the RFI provide a general indication of current practices in controlling occupational bloodborne pathogen exposure, experiences with safer medical devices, and perceptions of the most effective means of reducing infection risks. The number of responses received, as well as the opinions expressed by respondents, indicate that OSHA's concern with this issue is shared by many affected parties.
Sixteen questions were included in the RFI to provide a basis for composing a response (see Appendix A). In some instances, respondents addressed the same or similar issues in their responses to different questions. For example, some respondents discussed obstacles to the adoption of safer medical devices in answer to the question about the device selection process in their workplace. Other respondents discussed the same obstacles in reply to the question specifically directed at identifying obstacles. In addition, many respondents provided information and comment but did not specifically address the questions presented. In order to provide a coherent review, this summary is structured to address the broad issues raised in the responses, while generally following the content and sequence of the RFI questions.
Background
Percutaneous exposure to blood or other potentially infectious materials may pose a risk to the health of exposed individuals from the infectious agents that these materials may contain. The primary agents of concern in current occupational settings are human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). Other bloodborne pathogens include those responsible for syphilis, malaria, dengue, babesiosis, brucellosis, leptospirosis, Creutzfelt-Jacob disease, and Colorado tick fever (Exs. 3-172GG, 3-274C).
Recognizing the threat to the health of workers whose duties involve exposure to blood or other potentially infectious materials, OSHA issued the Bloodborne Pathogens Standard on December 6, 1991, to control occupational exposure (56 FR 64004). The standard requires that engineering and work practice controls be used to eliminate or minimize employee exposure to blood or other potentially infectious materials. These provisions can be found at 29 CFR 1910.1030 (d)(2)(i-ii):
Engineering and work practice controls shall be used to eliminate or minimize employee exposure. Where occupational exposure remains after institution of these controls, personal protective equipment shall also be used.
Engineering controls shall be examined and maintained or replaced on a regular schedule to ensure their effectiveness.
OSHA's Compliance Directive, CPL 2-2.44C, Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens Standard, establishes criteria to guide compliance officers in enforcing these provisions. The Directive states:
- Citations shall be issued if engineering or work practice controls are not used to eliminate or minimize employee exposure.
- While employers do not automatically have to institute the most sophisticated engineering controls (e.g., needleless IV connectors, self-sheathing needles), it is the employer's responsibility to evaluate the effectiveness of existing controls and to review the feasibility of instituting more advanced engineering controls.
Percutaneous injuries continue to be a concern in work settings where employees are exposed to bloodborne pathogens. Consequently, OSHA is interested in strategies for reducing percutaneous injury rates that have been successfully implemented in workplaces. This interest led the Agency to publish a RFI covering specific aspects of percutaneous injury prevention. The 16 questions in the RFI solicited information on the types of work settings where such injuries occur; percutaneous injury surveillance; use, evaluation, and effectiveness of control methods; and economic factors associated with the control of percutaneous injuries.
Responses to the Request for Information
OSHA has received 396 responses to the RFI. Included were comments provided by more than 300 individual health care facilities, representing over 170,000 employees with occupational exposure to blood or other potentially infectious materials in 47 states and Puerto Rico. A broad variety of facilities were represented, including nursing homes, clinics, and acute care, tertiary care, rehabilitation, and pediatric hospitals (e.g., Exs. 3-4, 3-58, 3-89, 3-92, 3-195, 3-202, 3-223, 3-241, 3-317, 3-321, 3-373). Facilities were located in both rural and urban areas and included teaching as well as non-teaching institutions. The responding facilities ranged in size from an outpatient nuclear cardiology lab with seven employees to a medical center employing 22,000 workers (Exs. 3-297, 3-364). Several organizations submitted combined responses on behalf of members representing over 130 additional healthcare facilities (Exs. 3-177, 3-258, 3-272E, 3-372). Also responding were individual healthcare workers, researchers, unions, educational institutions, professional and industry associations, and manufacturers of medical devices (e.g., Exs. 3-6, 3-13, 3-41, 3-45, 3-55, 3-128, 3-163, 3-172, 3-173, 3-179, 3-190, 3-266, 3-276, 3-387).
Surveillance
Over 98% of healthcare facilities responding to the RFI indicated that a system is employed in their facilities to record all reported needlesticks and other percutaneous injuries involving exposure to blood or other potentially infectious materials (hereinafter referred to as "injuries" or "exposure incidents"). Exposure tracking is universally reported to begin with a report for each exposure incident. The incident reports vary in scope and detail but generally record the individual injured, the device, and the circumstances surrounding the exposure incident (e.g., Exs. 3-85, 3-198, 3-282, 3-284). Surveillance systems often serve as the basis for identifying personnel, procedures, areas, and devices associated with a high risk of injury and subsequent infection; aiding in the determination of appropriate interventions; and evaluating the effectiveness of new devices (e.g., Exs. 3-15, 3-42, 3-160, 3-200, 3-281). Also, the system is sometimes used as a tool for managing workers' compensation insurance recordkeeping requirements or for managing post-exposure prophylaxis (e.g., Exs. 3-57, 3-160, 3-220). The sophistication of these surveillance systems varies considerably. Many facilities, particularly
smaller facilities, simply compile incident reports which may then be reviewed by individuals or committees with responsibility for infection control, employee health, or device selection (e.g., Exs. 3-30, 3-38, 3-81, 3-86, 3-181). Larger facilities often employ computer-based systems for this analysis (e.g., Exs. 3-31, 3-69, 3-104, 3-252).
The Exposure Prevention Information Network (EPINet) is a surveillance system reportedly acquired by 1500 healthcare facilities in the United States (Ex. 3-172). This system has established standardized incident report forms for sharp object injuries and for other exposures to blood and bodily fluids, as well as software for compilation and analysis of these data. Data from a group of approximately 70 hospitals are aggregated in a single database. This database serves as a resource for comparing and contrasting injury rates and exposure mechanisms recorded elsewhere. It also assists in identifying trends that may not be apparent from examination of smaller populations of exposed employees. The Centers for Disease Control and Prevention (CDC) established a second data sharing network, the National Surveillance System for Health Care Workers (NaSH). Other computerized tracking systems have been developed by individual facilities, insurance companies, and other private concerns.
Injury Rates
Responses to the RFI support the generally recognized fact that not all injuries sustained by employees are reported to employers (Exs. 3-278SSSS, 3-373E). The reasons for this problem are numerous. One reason appears to be a perception that a low risk of infection is associated with certain types of injuries and/or patients (Exs. 3-373E, 3-274C). Other hindrances to reporting noted in responses include employee lack of knowledge of appropriate procedures to follow after injury is incurred; time constraints; and the fact that some facilities require remedial training if a review of the circumstances surrounding the injury reveals improper employee procedures or work practices as a contributing factor to the injury (Exs. 3-108, 3-274, 3-373E).
The rate of underreporting is difficult to ascertain and information in the docket indicates that this rate may vary among facilities. For example, a collaborative study between the CDC and six hospitals indicated that 46% of injuries went unreported by healthcare workers (Ex. 3-278SSSS); surveys conducted in six EPINet hospitals disclosed that 39% of exposure incidents were not reported (Ex. 3-172V); and results of a survey conducted in one hospital indicated that 59% of injuries were not reported (Ex. 3-373E).
In the RFI, OSHA asked facilities to report their sharps injury rate. To allow comparison of the data, the Agency asked that respondents use OSHA's standard formula for calculating injury rates; this formula expresses the sharps injury rate in terms of the number of injuries per 100 full-time equivalent workers (FTEs) with occupational exposure. OSHA's intent was to allow comparison of respondent injury rates and to identify an average injury rate and injury rate trends among the respondents. However, several confounding factors were identified when OSHA attempted to perform such an analysis. For example, many of the responding facilities apparently do not normally calculate injury rates or use a different methodology for measuring rates. Respondents expressed injury rates per patient days, per occupied beds, or per number of devices used (e.g., Exs. 3-38, 3-101, 3-164, 3-177, 3-184, 3-188, 3-389). A number of institutions stated that they record injuries for all individuals, even if those individuals are not employees of the facility (e.g., students, self-employed physicians). One respondent commented that she was not able to calculate an injury rate using OSHA's formula because there was no way to determine the number of hours worked by such non-employee individuals (Ex. 3-131). In addition, facilities used different criteria to determine what exposure incidents to include when computing the injury rate. Some facilities, for example, included injuries from non-contaminated sharps, while others included skin and mucous membrane exposures along with percutaneous exposures in computing a rate (e.g., Exs. 3-123B, 3-256).
Most respondents attempted to provide a rate based on percutaneous injuries from contaminated sharps among FTEs with occupational exposure. In some cases, however, the respondent did not have the information necessary to provide a rate in this form. In addition, several factors complicated the examination of injury rate trends over time. More specifically, in many instances the respondent had not yet computed the year-to-date rates for 1998, while in other cases historical data were not available or were available only for a limited span of time. Still, review of the responses led to the general observation that percutaneous injury rates appear to be declining. The success reported in many facilities that implemented intervention efforts to reduce injuries during this time period seems to qualitatively support this observation. For example, data submitted by the Department of Veterans Affairs (VA) indicates a decline in the number of injuries recorded in VA healthcare facilities from fiscal year 1992 through fiscal year 1996 and is noteworthy because the decline occurred against a backdrop of progressively improved reporting of injuries (Ex. 3-123A).
The total number of injuries sustained annually in the United States is unknown. The lack of data for non-hospital settings appears to be a particularly troublesome factor in arriving at a national injury estimate. The International Healthcare Worker Safety Center (IHWSC) provided working estimates of the number of injuries based on 1996 EPINet data. These data indicate that 30 percutaneous injuries were reported per 100 occupied hospital beds for that year. Applying this number to 600,000 occupied hospital beds in the U.S. resulted in a figure of 180,000 reported injuries. According to surveys conducted in six EPINet hospitals in 1996-1997, 39% of incidents were not reported. Adjusting for this factor led to an estimate of approximately 295,000 injuries in hospitals annually. Making a further adjustment to account for healthcare workers employed outside of the hospital setting resulted in a final IHWSC estimate of approximately 590,000 injuries annually (Ex. 3-172V). The number of infections resulting from these injuries has also been estimated (Exs. 1-5, 3-172V).
Recording of Injuries
Recording of injuries in the OSHA 200 log is inconsistent among responding facilities. The criteria for recording bloodborne pathogen exposure incidents are clarified in CPL 2-2.44C, which states:
J. Recording of Exposure Incidents. For OSHA 200 recordkeeping purposes, an occupational bloodborne pathogens exposure incident (e.g., needlestick, laceration, or splash) shall be classified as an injury since it is usually the result of an instantaneous event or exposure. It shall be recorded if it meets one of the following recordability requirements:
- The incident is a work-related injury that involves loss of consciousness, transfer to another job, or restriction of work or motion.
- The incident results in the recommendation of medical treatment beyond first aid (e.g., gamma globulin, hepatitis B immune globulin, hepatitis B vaccine, or zidovudine) regardless of dosage.
- The incident results in a diagnosis of seroconversion.
Not all injuries meet these criteria, and some disparity between facilities in the proportion of injuries that are recorded is expected even when injuries are recorded correctly. A hospital serving a patient population with a high HIV infection prevalence, for example, would likely have a larger percentage of recorded injuries than a hospital serving a population with a low HIV infection rate because the administration of zidovudine may be recommended after an HIV-associated injury. The wide divergence in the proportion of such injuries that are entered on the log at different facilities, however, appears to indicate an apparent misunderstanding or misapplication of the recording criteria. This situation was noted by the American Association of Occupational Health Nurses (AAOHN). After a survey of members reported a range of zero to 100% of injuries being recorded on the 200 log, the AAOHN commented that misunderstandings about the recordability of these types of injuries may account for the wide range (Ex. 3-272A). Of the 190 responses to the RFI providing both the total number of injuries and the number of injuries entered in the OSHA 200 log at their respective facilities, 28 (15%) did not record any injuries and 67 (35%) recorded all injuries. A further breakdown is presented in Chart 1:
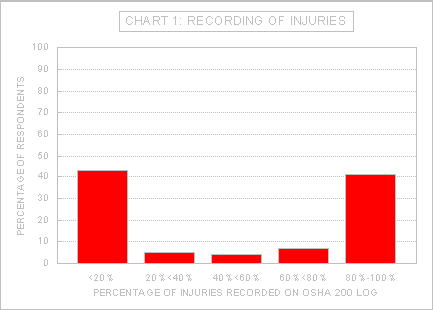
Source: OSHA Directorate of Health Standards
Selection and Evaluation of Devices
Evaluation of exposure controls and acquisition of safer medical devices or training varies among respondents. The comments indicate that these decisions typically involve individuals or multi-disciplinary committees with responsibility for employee health, infection control, risk management, and materials management (e.g., Exs. 3-28, 3-50, 3-371). Decision-makers use injury data to develop alternatives to high risk procedures. These decisions are also made after consultation with staff and peers who may be able to provide guidance (e.g., Exs. 3-100, 3-118, 3-153, 3-169).
Other than the ability of a device to perform the function for which it was designed, factors commonly affecting the recommendation for safer medical devices are the effects on patient care, expected effectiveness in reducing injuries, anticipated cost, ease of use, staff preference, compatibility with other devices and systems, and, sometimes, facility contractual purchasing agreements (e.g., Exs. 3-91, 3-97, 3-104, 3-116, 3-135, 3-160, 3-366). Some respondents conduct trials in limited areas or applications to gauge product efficacy before reaching a final decision concerning adoption of the product on an expanded scale (e.g., Exs. 3-49, 3-56, 3-62, 3-88, 3-371).
A fairly common difficulty expressed by respondents was the inability to identify specific devices that may be appropriate for adoption in their facilities (e.g., Exs. 3-18, 3-60, 3-188, 3-202, 3-225, 3-274). Some respondents reported that the wide array of available devices, often combined with relatively little evidence supporting the safety claims for those devices, complicates the selection process (e.g., Exs. 3-177, 3-250, 3-257, 3-313). However, general information to assist in selecting devices was submitted to the docket. Researchers have suggested that safer medical devices should (Ex. 3-278Z):
- Provide a barrier between the hands and the sharp after use;
- Allow or require the worker's hands to remain behind the sharp at all times;
- Be an integral part of the device and not an accessory;
- Be in effect before disassembly and remain in effect after disposal to protect downstream
workers; and
- Be simple and self evident to operate and require little or no training to use effectively.
The U.S. Food and Drug Administration (FDA) has agreed that for recessed needle systems, devices with these characteristics have the potential to reduce the risk of injury (Ex. 1-17). FDA is continuing to work on design and performance criteria for safer medical devices (Ex. 3-248). In addition, the Training for Development of Innovative Technologies Project (TDICT) has focused on the selection and evaluation of safer medical devices. TDICT is a collaborative effort of healthcare workers, product designers, and industrial hygienists based at San Francisco General Hospital and funded through a cooperative agreement with the CDC. This group has developed safety feature evaluation forms for 13 different types of devices and is currently developing protocols for the testing of devices (Ex. 3-270). The docket also contains published reviews and studies of specific products (e.g., Exs. 3-12, 3-278, 3-336, 3-342). However, the comments of the respondents indicate that such information is not reaching the appropriate parties or is insufficient to convince evaluators that a particular product is effective in protecting workers (e.g., Exs. 3-43, 3-159, 3-208).
Use of Safer Medical Devices
The vast majority of responding facilities indicated that they had adopted safer medical devices at least to some extent. IV line access is the most common application for safer medical devices, with over 87% of the respondents who provided information on this topic reporting their facilities used safer medical devices for this procedure (e.g., Exs. 3-16, 3-83, 3-258, 3-267, 3-307). Use of safer medical devices for other procedures, such as phlebotomy, is less extensive, as seen in Chart 2. A number of respondents also cited use of other devices such as blunt suture needles and magnetic trays for passing sharp instruments (e.g., Exs. 3-87, 3-143, 3-146, 3-289).
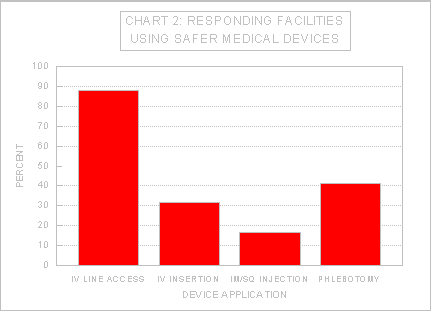
Source: OSHA Directorate of Health Standards
However, although these figures indicate that the respondents use safer medical devices in the applications listed, they do not represent the extent of safer medical device use. In many instances, respondents have not adopted safer medical devices facility-wide or have not replaced all conventional devices for a particular application.
The VA reported that in 1996, among 156 VA facilities, 96% used safer medical devices for IV delivery, 69% used safer medical devices for blood collection, 54% used safer medical devices for injections, and 53% used safer medical devices for IV insertion (Ex. 3-123A). In addition, the Healthcare Association of New York State surveyed over 100 acute care facilities and found that 99% have adopted safer IV equipment, 47% have adopted safer syringes, and 47% have adopted safer phlebotomy devices (Ex. 3-177).
Safer devices have been estimated to comprise 65% of the total market for IV line access systems, greater than 40% of the market for winged steel needles, and less than 10% of the markets for vacuum tube blood collection needles and IM/SQ needle/syringes (Exs. 3-74, 3-172). These figures are somewhat lower than the facility-adoption figures presented in Chart 2, because safer medical devices do not always replace conventional devices in all areas and applications within a facility. Also, facilities responding to the RFI may be more progressive in implementing safer medical devices than facilities in general.
Based on anecdotal reports in the docket, overall use of safer medical devices appears to be increasing as more facilities convert from conventional to safer devices (e.g., Exs. 3-38, 3-75, 3-103, 3-288, 3-351). With regard to a specific device, the conversion rate from standard IV catheters to IV catheters with safety features is presented in Chart 3 (Ex. 3-71).
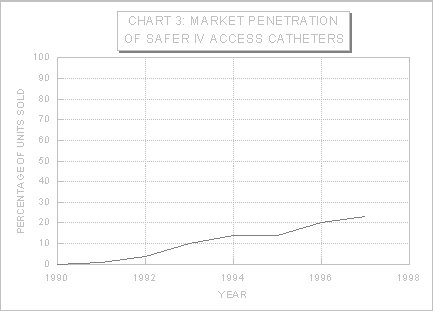
Source: OSHA Directorate of Health Standards, based on data provided by Johnson & Johnson Medical (Ex. 3-71)
However, as the preceding paragraph demonstrates, adoption of various other types of safer medical devices is not occurring at an equivalent rate. That is, facilities are adopting some types of safer medical devices more readily than others (e.g., Exs. 3-16, 3-83, 3-258, 3-267, 3-303).
In addition, even though the development of safer medical devices has continued over a number of years, some respondents stated that they were not able to find any suitable substitutes for conventional devices for certain functions. This concern was particularly noted in dentistry and, to a lesser extent, in pediatric applications (e.g., Exs. 3-18, 3-60, 3-188, 3-202, 3-225, 3-266, 3-274).
Training
Respondents repeatedly emphasized the necessity of effective training and education whenever safer medical devices are implemented, as a means of increasing awareness both of potential infection hazards and appropriate work practices to mitigate those hazards. Safer medical devices commonly require healthcare workers to learn new techniques and work practices. Training ordinarily occurs during the orientation of new hires, annually thereafter, and with the introduction of new devices (e.g., Exs. 3-92, 3-137, 3-157A). Some facilities also conduct competency testing and/or require remedial training when improper practice is observed or an exposure incident occurs (e.g., Exs. 3-60, 3-84).
For the most part, respondents indicated that new safer medical devices were readily accepted and correctly used by staff members when introduced into their facility (e.g., Exs. 3-1, 3-10, 3-88, 3-341, 3-347, 3-369). A number of respondents, however, encountered some staff resistance when new devices required staff members to adopt new techniques, or when staff members perceived that use of the device had an adverse effect on patient care (e.g., unsuccessful attempts at venipuncture; additional time required to perform procedure) (e.g., Exs. 3-50, 3-79, 3-99, 3-133). These factors were particularly notable with the adoption of some safer IV catheters. Acquiring proficiency in the use of these devices apparently requires a considerable modification of previous technique, and some respondents mentioned that successful catheter insertion required a number of attempts. As a way of addressing this resistance, many respondents felt that staff involvement in the selection process plays an important role in the acceptance and proper use of safer medical devices (e.g., Exs. 3-18, 3-42, 3-56, 3-88, 3-324, 3-355). Also, respondents cited adequate training as a critical element in attaining staff support for the continued and appropriate use of safer medical devices (e.g., Exs. 3-2, 3-118, 3-194, 3-291, 3-361).
Effectiveness of Safer Medical Devices in Reducing Injury Rates
Respondents to the RFI indicated widespread success in reducing percutaneous injury rates through the introduction of safer medical devices. Nearly every facility noted a reduction in injuries with some safer device. The success that has been encountered with safer medical devices has also been substantiated by published studies. This research provides evidence that a variety of devices have proven effective in reducing percutaneous injuries:
- In a collaborative study by the CDC and six hospitals, use of safer medical devices for phlebotomy procedures (a resheathable winged steel needle; a bluntable vacuum-tube blood-collection needle activated while in the patient's vein; and a vacuum-tube blood-collection needle with a hinged recapping sheath) was found to substantially reduce injuries associated with those procedures while having minimal clinically apparent adverse effects on patient care. Injury rates for the three devices examined were 23% to 76% lower than injury rates for the conventional devices they replaced (Ex. 3-278SSSS).
- In a study of 26 facilities that had purchased a combined 86,300 automated retraction syringes over a 12-month period, no injuries were reported from use of these devices. A survey of the facilities indicated that, in the year prior to this purchase, one needlestick was reported per 3,288 syringes purchased (Ex. 3-336A).
- In a collaborative study by the CDC and three hospitals, use of blunt suture needles was associated with significant reductions in injury rates, with minimal clinically apparent adverse effects on patient care and general acceptance reported by surgeons. Conventional curved suture needles were associated with 1.9 percutaneous injuries per 1000 needles used (56 injuries per 28,880 conventional curved suture needles used); blunt needles were associated with zero injuries per 1000 needles used (0 injuries per 6139 blunt suture needles used) (Ex. 1-16).
- In three hospitals using conventional and safer IV catheters concurrently, the safer medical devices were shown to reduce the associated incidence of injury by 84% when compared to conventional devices. Historical data from one of these facilities on injury rates experienced with conventional catheters prior to implementation of educational safety programs and improved sharps disposal systems indicated that these practices had already achieved a 59% reduction in injury risk, for a combined 94% reduction for all measures combined (Ex. 3-172R).
Not all respondents, however, reported unqualified success after the adoption of safer medical devices. Some indicated that particular devices, or devices used in certain applications, were not effective. In a few instances, devices designed to be safer were found to increase the number of injuries. E. Cuny and R. Fredekind of the University of the Pacific School of Dentistry reported a negative experience with self-sheathing dental needles. Even though a manufacturer's representative provided hands-on training to all individuals in the use of the device, an increase in needle-related injuries occurred. However, these respondents noted success with other intervention measures. More specifically, they noted that use of disposable scalpels led to a decrease in associated injuries, prohibiting the transfer of an uncapped dental syringe between assistant and dentist resulted in a slight reduction in injuries, and modified placement of the dental handpiece resulted in a dramatic reduction in bur-related injuries (Ex. 3-266).
Other factors that influence the successful implementation of safer medical devices include continued evaluation of devices and the allotment of sufficient time for adequate device evaluation. More specifically, some respondents found it necessary to replace the device originally selected with a more suitable device. P. Macheichok of Hess Memorial Hospital indicated that after a retracting lancet was put into service at that facility, injuries continued to occur due to protrusion of the lancet during disposal. Use of a different retracting lancet was effective in eliminating lancet-associated injuries (Ex. 3-235).
In addition, successful implementation of a device may simply require that the evaluators give staff sufficient time to become accustomed to the device and any associated new techniques. N. Lorenzoni of St. Vincent Hospital, after reporting no problems with conversion to other safer medical devices, went on to state:
IV catheters have been a more difficult process. There is a change in technique, the device is bigger and more cumbersome, and proficiency of IV insertions has suffered. However, practice and use of the device has been the key factor. Persistence with use has paid off. Those nurses who have used the product the longest are quite comfortable with it and would never go back to use of a device that is not safe (Ex. 3-100).
The adoption of safer medical devices in general has not meaningfully affected the delivery of patient care among respondents. However, a small number of responses described positive or, to a lesser extent, negative impacts on patient care. Typical of the positive responses was one by D. Coppage of Brookwood Medical Center, who wrote:
. . . when employees have a safe working environment including appropriate safety devices, the patient benefits from having a confident, interested staff, thereby providing a higher quality of care. Also, these devices prevent cross contamination from one patient to another (Ex. 3-265).
Also, the Healthcare Association of New York State stated that, among over 100 acute care facilities surveyed by that organization, 53% felt that the use of safer medical devices has made procedures safer for both patients and employees (Ex. 3-177).
In contrast, several respondents felt that particular devices were more cumbersome to use andresulted in slower performance (e.g., Exs. 3-103, 3-110, 3-136). Others indicated that certain devices, particularly some IV catheters, might be more painful for the patient (e.g., Exs. 3-133, 3-164, 3-170). M. Cohen of Kaiser Permanente wrote:
Some of the sharps safety devices have adversely affected patient care. For example, patients and medical professionals have reported that some safety syringes cause unnecessary pain. Some blood draw devices and safety catheters we tested required additional venipunctures; they also increased pain and caused hematomas (Ex. 3-115).
The majority of respondents, however, indicated that the use of safer medical devices had no observed impact on patient care.
Obstacles to Adoption of Safer Medical Devices
The two most frequently cited impediments to the introduction of safer medical devices were the increased cost associated with these devices and staff resistance to changes in the devices used. As noted previously, safer medical devices often require adjustments in technique, and a number of respondents noted that staff members are often reluctant to revise practices to which they have become accustomed.
Respondents also noted equipment compatibility as an obstacle in certain situations (e.g., Exs. 3-116, 3-118, 3-168). With the broad array of devices being used in healthcare settings, it is necessary to ensure that devices will function in unison when necessary. A safer blood collection tube holder, for example, must be compatible with the blood collection tubes used by the facility. In some situations compatibility issues may extend beyond the facility. Selection of devices in an emergency room, for example, may be influenced by the devices used by emergency medical service personnel, in order to ensure a smooth transition in the delivery of patient care (e.g., Exs. 3-29, 3-84, 3-293, 3-302, 3-315).
A number of respondents indicated that contractual purchasing agreements entered into by a facility limited the alternatives available for addressing exposure hazards (e.g. Exs. 3-92, 3-93, 3-108, 3-170). According to some respondents, agreement by the facility to purchase supplies from a limited number of vendors through a group purchasing organization in return for a price discount restricts the range of devices considered in the selection process. K. Korte and J. Bailey of Arrowhead Community Hospital and Medical Center wrote:
One obstacle [to purchasing safer medical devices] is that of our purchasing contracts, or the need to purchase from certain companies who we are contracted with. This has not been a big problem, but it certainly could be, because a maximum of 20% of our purchases can be outside the current contracts, so very careful planning and purchasing must be done. If a certain device is desired and cannot be purchased under the current contract, it is definitely more difficult to bring that item into the hospital (Ex. 3-291).
T. Shaw of Retractable Technologies, a device manufacturer, described the problem faced by his firm in attempting to sell safer medical devices:
Our employees and representatives have been told that they may not show our products to healthcare workers in hospitals across the country. The purchasing personnel in facilities consistently indicate that they are prevented from looking at or evaluating needle products that are not approved by their group purchasing organization (Ex. 3-336).
Several respondents commented that in certain situations safer medical devices are not available or are not a practical alternative to conventional devices (e.g., Exs. 3-18, 3-60, 3-188, 3-202, 3-225, 3-274). However, the data indicate that in many situations safer medical devices are not being used, even though evidence is available that such devices represent a reasonable alternative to conventional devices and an effective means of controlling exposure to bloodborne pathogens. For example, seven years ago, the FDA issued a safety alert strongly urging that needleless or recessed needle systems replace hypodermic needles for accessing IV lines (Ex. 1-17). Numerous facilities responding to the RFI and reports in the literature support the ability of facilities to institute this control measure and verify its success in reducing injuries. However, the gathered information indicates that a substantial portion of IV line access is still performed using conventional devices (e.g., Exs. 3-94, 3-112, 3-149, 3-172). Continued use of glass capillary tubes (commonly used when centrifuging a blood sample for hematocrit determination) is another example of users not implementing safer medical devices that are available. The FDA recommends that safer alternatives to glass capillary tubes be considered. More recently, FDA, OSHA, and the National Institute for Occupational Safety and Health (NIOSH) issued a joint safety advisory recommending that users consider blood collection devices that are less prone to accidental breakage (Ex. 1-18). However, although safer alternatives such as plastic tubes and mylar-wrapped tubes are available, glass capillary tubes are currently estimated to be associated with approximately 2,800 percutaneous injuries annually (Ex. 3-172S).
Cost Issues
Most of the comments received in response to the RFI did not provide detailed cost information or even specifically address the issue of costs. Some respondents addressing the cost issue simply made general statements reflecting a perception that safer medical devices were associated with increased costs. Nevertheless, several dozen respondents did provide detailed cost data for an array of different products.
Cost data were submitted from a variety of sources. Many hospitals shared data from their own experiences, providing estimates of overall costs as well as incremental cost increases associated with the implementation of various devices (e.g. Exs. 3-281, 3-355, 3-366, 3-367). Data were also provided by device manufacturers and suppliers that showed differences in unit costs as well as overall impacts on establishments adopting new technologies. Additional research and data were made available by the IHWSC (Ex. 3-172) and government sources, including a report completed by the State of California Department of Health Services regarding proposed regulatory changes in that state (Ex. 3-273).
Nearly all of the respondents addressing the question of costs reported that safer medical devices cost more than conventional devices. The extent of the cost increase depends on the specific device purchased, the type of device being replaced, the quantity of devices used, and on other changes, such as in work practices, that may be associated with the implementation of a new device. On a per-unit basis, the prices of safer medical devices tend to be substantially higher than those of conventional devices. However, the total additional costs of adopting safer medical devices for a typical hospital appear to represent only a small fraction of health care costs. Many hospitals have already switched to safer versions of sharps products for a range of different applications.
A number of respondents provided details about the costs associated with specific sharps devices and their experience with the implementation of safer medical devices. For example, one respondent stated that a new protective catheter implemented to reduce needlestick risk costs $1.87 versus $0.71 for the cost of a conventional catheter (Ex. 3-321). According to another respondent, the added cost for products with safety protection features ranges from 7 to 15 cents for a syringe/needle combination and from 15 to 30 cents for a blood collection needle or set, to about 70 cents for an intravenous catheter. If a 300-bed hospital has already switched to a needleless IV access system but does not purchase any sharps with enhanced protection features, the annual incremental cost to make these products available would be less than $75,000 (Ex. 3-278).
The IHWSC provided a comparison of the costs of some safer medical devices with their conventional counterparts (Ex. 3-172). This information is presented in Table I:
| TABLE I: INCREMENTAL COST OF SAFER MEDICAL DEVICES
PER FACILITY |
| Purpose of Device |
Device |
Unit Price (Conventional) |
Unit Price (Safer) |
Annual Cost for 250-300 Bed Hospital |
| Conventional |
Safer |
Incremental |
| Venous Blood Draw
|
Vacuum Tube Phlebotomy Needle |
$0.10 |
$0.33 |
$6,500 |
$22,000 |
$15,500 |
| Butterfly Needle |
$0.65 |
$0.90 |
$11,000 |
$15,000 |
$4,000 |
| IV Access |
IV Catheter |
$0.75 |
$1.75 |
$25,000 |
$58,500 |
$33,500 |
| IM/SQ Injection/Fluid Transfer |
Hypodermic Needle/Syringe |
$0.05 |
$0.25 |
$16,500 |
$83,500 |
$67,000
|
Source: Bioplexus, Inc., provided by the International Healthcare Worker Safety Center (Ex. 3-172MM)
According to the Economic Impact Statement submitted with the proposed sharps protection regulation in California on November 16, 1998, the requirements of the regulation are expected to impose average annual costs of about $1,770 on a typical affected business. The total number of affected businesses in California is estimated as 84,000, and the types of businesses affected include: hospitals, clinical laboratories, blood banks, nursing care facilities, home health care providers, physicians' offices, dentists' offices, emergency medical services, research laboratories, and funeral services. The total annual costs of the proposed regulation for establishments in California are estimated at $186 million (Ex. 3-273H).
Most respondents also felt that the use of safer medical devices generally reduced the risk of percutaneous injuries among health care workers. Hospitals consistently reported that needlestick injuries suffered by employees involve significant costs to the employer. These costs, which translate directly into savings resulting from reductions in risk, involve those associated with time and resources for testing, treatment, counseling, administrative work, and adverse productivity effects (e.g., Exs. 3-271, 3-278, 3-327, 3-351).
Estimates submitted to the record of the average baseline expenses associated with needlestick injuries or other blood exposures ranged from under $500 (e.g., Exs. 3-271, 3-253) to over $3,000 (Ex. 3-273), with most estimates falling between $500 and $1,000 (e.g., Exs. 3-277, 3-285, 3-347, 3-365, 3-327, 3-278 3-321). These respondents generally acknowledged that the costs associated with a needlestick injury could be dramatically higher if the injury resulted in an infection, such as with hepatitis or HIV. One respondent stated that in such cases, the cost could be several hundred thousand dollars or even millions of dollars (Ex. 3-327).
Sharps Disposal Issues
Many of the respondents described similar problems with sharps disposal containers. Overfilling is a frequently reported problem. Respondents often associated this problem with containers that were too small to accommodate the volume of sharps generated in the immediate area of use, employee inability to see the contents of the container in order to determine the remaining capacity, or lax container maintenance procedures (e.g., Exs. 3-7, 3-61, 3-75, 3-181, 3-302, 3-365).
Another concern related to the location and placement of containers. Some respondents noted a lack of containers in areas where they are needed as well as difficulties resulting from placement of the container at an inappropriate height (e.g., Exs. 3-62, 3-83, 3-185, 3-250, 3-309). Respondents also noted that some containers had problems in design or construction (e.g., Exs. 3-75, 3-184, 3-235, 3-237, 3-366). However, most facilities were able to address these problems by using a different container. In addition, some respondents mentioned using materials developed by NIOSH to provide guidance on the selection, evaluation, and use of sharps disposal containers.
Suggested Approaches to Reduce Infection Risk
The great majority of respondents included employee education and training along with use of safer medical devices when they described the most effective means of reducing the number of injuries. They repeatedly emphasized these factors, and commonly referred to education and the use of safer devices in combination. Baptist Memorial Hospital-Lauderdale, for example, wrote:
The most effective means of reducing sharps injuries is education for the employee. Safety devices are an asset to the employee, but they [the employees] must receive the proper inservice and education (Ex. 3-369).
N. King of Florida Power & Light/Whole Health Management commented:
The use of the proper device is the most important aspect of a safe program. Continuous training as well as safety awareness in the workplace is also imperative to the elimination of injuries (Ex. 3-112).
S. White of Carraway Northwest Medical Center maintained:
Education of employees cannot be stressed enough. Safety devices are one way of reducing sharps injuries; however, they cannot replace education. Work practices are just as, if not more important than safety devices (Ex. 3-89).
Respondents also identified other factors they felt were important in reducing injuries. These factors include effective surveillance of injuries combined with targeting of injury prevention measures, employee involvement in determining appropriate interventions, an effective sharps disposal system, and adherence to proper work practices (e.g., Exs. 3-69, 3-148, 3-156, 3-242, 3-324). Many respondents appear to consider a programmatic approach the most effective approach to reducing injuries. Respondents reported that surveillance data are commonly used for both hazard identification and evaluation of program effectiveness; safer medical devices and work practices are used for hazard control; and education and training are stressed (e.g., Exs. 3-324, 3-355); these components are all elements of an injury prevention program. In addition, respondents often stressed that employee involvement is integral to success. Speaking about employee involvement, the American Nurses Association wrote:
Nurses and other frontline healthcare workers must be involved in the analysis of exposure data and the evaluation, selection and implementation of safer needle devices to eliminate exposure to hazards. Where implementation of safer devices has been successful, it has been where the frontline healthcare workers have been involved (Ex. 3-274).
With regard to a programmatic approach, B. Staats and L. Miller of the Mayo Clinic cited the elimination of needles, use of devices with passive safety features, enforcement of safe work practices, and education as vital to the success of injury prevention efforts. They wrote:
All of these control methods, engineering, enforcement, and education are required to reduce the number of needlesticks. One method alone is not enough. New devices, work practices and individual behaviors have to be monitored and evaluated. The best people to accomplish improvements in a specific workgroup are members of that group dedicated to improving their workplace safety (Ex. 3-297).
Some respondents considered a total conversion to safer medical devices to be desirable. The International Healthcare Worker Safety Center wrote:
The International Healthcare Worker Safety Center is in favor of measures that would result in a complete transition from conventional to safety devices in the U.S. healthcare workplace. We very much hope that this RFI will result in OSHA's taking concrete steps to bring this about, thus creating a safer work environment for American healthcare workers in every state (Ex. 3-172).
A number of other respondents advocated action by the Agency to require employers to make safer medical devices available to employees. Some urged OSHA to include other elements of an injury prevention program that they considered necessary in any Agency action. The Service Employees International Union (SEIU) cited a number of conventional medical devices for which safer substitutes are currently available and stated:
. . . use of such inherently dangerous devices when safer alternatives are available in the marketplace should result in immediate OSHA citations.
The SEIU also commented:
We have concluded that the two most critical elements in the success of any needlestick prevention program are extensive employee involvement - usually through the formation of a joint labor management needlestick prevention committee, and adequate training - including hands-on training - on the use of newer, safer technologies (Ex. 3-163).
The American Federation of Government Employees echoed support for mandatory availability of safer medical devices, along with employee involvement in the selection of devices and training in their use:
From a moral and ethical point of view, it is inconceivable that health care workers should be at risk of needlesticks when the technology to prevent these hazards is currently available (Ex. 3-179).
Support for mandatory availability of safer medical devices was expressed by other respondents, including the American Federation of State, County and Municipal Employees (Ex. 3-346), and the New York State Public Employees Federation (Ex. 3-244). Others, including the United Food and Commercial Workers International Union (Ex. 3-322), the SEIU, as seen above (Ex. 3-163), and the American Nurses Association (Ex. 3-274), supported this provision as well as a requirement for the recording of all exposure incidents.
Some respondents qualified their support for safer medical devices. A number of respondents mentioned the importance of considering the efficacy of safer medical devices before using them in the workplace. The Healthcare Association of New York State wrote:
New safety devices should be implemented only after careful risk assessment, followed by pilot testing and evaluation of selected devices to determine efficiency and efficacy for health care workers as well as their impact on patient safety and well-being (Ex. 3-177).
Kaiser Permanente expressed a similar sentiment:
The use of sharps safety devices is an important measure to reducing possible needlesticks and other percutaneous injuries, but the selection and implementation of such devices in the work place requires a disciplined approach that includes validation of the product's efficacy and its impact on patient care. Safety devices put in the work environment without proper evaluation can increase employee exposures and compromise patient safety (Ex. 3-115).
The Nassau-Suffolk Hospital Council maintained that hospitals need flexibility to address their own unique situations. The Council contends that education and compliance with safe practices may be more successful than use of specific products (Ex. 3-106). Similarly, C. Pelletier of St. Vincent Hospital wrote:
A safety device that may work in one organization may not work in another due to differences in populations served or services provided (Ex. 3-251).
Consideration of the distinctive needs of particular areas of practice was urged by the American Society of Anesthesiologists, who wrote:
The practice of anesthesiology, as with other medical specialties, requires unique clinical procedures which are different from those performed by other groups of healthcare workers such as nursing personnel. Therefore, implementation of specific devices or work practices must be evaluated by individual medical specialties and job categories before being formalized (Ex. 3-67).
In addition, the American Dental Association and the Academy of General Dentistry indicated that the practice of dentistry entails substantially different exposure risks than medical practice (Exs. 3-122, 3-132). These respondents indicated that percutaneous injuries were infrequent during dental procedures. They stated that many injuries are associated with dental burs and other solid dental tools for which safer substitutes are not available; safer syringes for dental applications have not always proven effective; and the needles used in dentistry have a more narrow bore than those used in medical procedures, resulting in a lower volume of blood exposure when injury does occur.
Summary
The preceding report summarizes the responses to OSHA's RFI on needlesticks and other percutaneous injuries. The responses related personal and institutional experiences with safer devices and work practices and include observations and viewpoints about percutaneous injury prevention. The responses indicate that many healthcare facilities already engage in injury surveillance and have adopted safer medical devices to some extent. Safer medical devices along with training and education are widely regarded as the most effective means of reducing injury rates. Employee involvement in the device selection and evaluation process is also often considered an important factor in successful efforts to reduce injuries. However, despite the demonstrated effectiveness and feasibility of safer medical devices in many situations, the increased costs associated with acquiring these devices, along with other obstacles, have limited their adoption, according to some respondents. A few respondents were concerned that a broad regulatory requirement for safer medical devices may not provide sufficient flexibility to address the clinical needs of patients or be the most effective means of protecting workers, while a number of respondents urged the Agency to place greater emphasis on the use of engineering controls in affected facilities as a means of providing an impetus to employers to adopt safer devices.
APPENDIX A
Questions Included in the Request for Information of September 9, 1998 (63 FR 48250)
- What is the type, size, and employment of your facility or work setting? OSHA solicits information on the type and size of your facility or work setting (e.g., 200-bed tertiary care hospital, 10-bed nursing home), the total number of employees, how many of these employees have the potential to sustain a needlestick or other percutaneous injury during performance of their job duties and, if possible, the job classification(s) of these employees.
- Does your facility have a surveillance system to track needlesticks and other percutaneous injuries? If yes, please state if your system includes tracking of needlesticks and other percutaneous injuries other than those that must be recorded on the OSHA 200 log. OSHA solicits information on systems being used to track needlesticks and other percutaneous injuries, if and how the gathered information is used, and any factors affecting the successful implementation of such systems.
- What is the total number of potentially contaminated needlesticks and other percutaneous injuries that have occurred in your facility in the past year and in previous years? OSHA solicits information on how many of these needlesticks and other percutaneous injuries were recordable on the OSHA 200 log and how many were non-recordable.
- What is the rate of injuries from potentially contaminated needles and other sharps in your workplace in the past year and in previous years? If possible, please express your response in terms of Injuries per 100 Workers according to the following formula:
(Number of Injuries from question # 3) x 200,000*
Rate = ----------------------------------------------------------
Hours Worked**
* Base for 100 equivalent full-time workers, working 40 hours per week,50 weeks per year.
** Includes hours worked by all full time, part time, or temporary workers covered by your bloodborne pathogens exposure control plan.
OSHA seeks information and comment on needlestick and other percutaneous injury rates and/or patterns associated with particular employee groups, work locations, procedures, or devices.
- What methods and criteria are used in your workplace to evaluate the effectiveness of existing exposure controls? If a system is used in your workplace for periodic review of the feasibility of instituting more effective engineering controls, please describe the system including the type of information obtained, how this information is applied, and how the appropriate individuals in your workplace become aware of the availability of new controls.
- Has any type of integrated percutaneous injury prevention program, as discussed above, been established in your workplace to reduce the incidence of needlesticks and other percutaneous injuries? If yes, OSHA solicits information and comment on the structure and content of this program (e.g., safer work practices, safer medical devices, training), the results achieved, and any specific problems and/or successes that have been encountered in the implementation and operation of the program.
- To what extent have devices designed to reduce the incidence of needlesticks and other percutaneous injuries been adopted in your workplace? Please provide any workplace- or industry-specific data you have available indicating the degree to which devices incorporating safety features have replaced standard devices, with specific information on the types (e.g., needleless IV connector, blunt suture needle) and brand or description of devices used; where such devices are used (i.e., specific locations, procedures, or employee groups); and any historical data indicating the rate at which your workplace has implemented safer medical devices over the years.
- On what basis are decisions made in your workplace concerning selection of safer medical devices? OSHA solicits information and comment on design and/or performance criteria being used to select safer medical devices and the basis for using the particular criteria; if and how percutaneous injury data are used in making selection decisions; if and how the opinions of the primary users of needles and other sharps are considered in selection decisions; how costs are considered in the selection process; and any other factors that influence selection decisions.
- Have new safer medical devices been readily accepted and correctly used when provided? OSHA seeks information and comment on factors influencing successful implementation of safer medical devices in the workplace.
- What provisions are made to ensure adequate training and education in the use of safer medical devices and/or safer work practices in your workplace? OSHA solicits information and comment on the effectiveness of training and education in reducing needlesticks and other percutaneous injuries, both relative to and in conjunction with the implementation of safer medical devices and/or safer work practices. Specific information is desired regarding program elements, successful and/or unsuccessful measures undertaken, and the method(s) by which results were measured.
- How effective are safer medical devices and/or safer work practices in reducingpercutaneous injury rates? OSHA seeks information and comment on the efficacy of safer medical devices and/or safer work practices in reducing injuries from needles and other sharps, including any data available that will aid in quantifying these results in total and/or for specific employee groups, work locations, procedures, devices or work practices; and the method(s) by which these data were obtained. OSHA is particularly interested in data regarding the percutaneous injury rates prior to implementing the device(s) and/or work practice(s), steps used in selecting and implementing the device(s) and/or work practice(s) in the work setting, and the percutaneous injury rates after implementation.
- Has use of safer medical devices and/or safer work practices in any way affected the delivery of patient care? If yes, please describe the effects and any data quantifying these effects.
- Based on observations in your workplace and your knowledge from other sources, please describe any obstacles that may be encountered relative to the selection, purchase, and effective implementation of currently available and new safer medical devices in the workplace, along with any specific information and comment you can provide detailing successful and/or unsuccessful methods of overcoming these obstacles.
- OSHA solicits information on the costs associated with the implementation of safer medical devices and any savings resulting from their use. Please provide specific information on the methods used to calculate these costs and savings.
- Please describe any problems associated with sharps disposal containers in your workplace, as well as successful and/or unsuccessful measures that have been undertaken to correct these problems.
- Based on experience in your workplace and your knowledge from other sources, what are the most effective means of preventing needlesticks and other percutaneous injuries? Please explain the basis for your opinion on this matter and provide any supporting evidence.
|
