2004 Progress Report: Fundamental Understanding and Performance Enhancement of Conductive Adhesive for Microelectronic Packaging Applications
EPA Grant Number: R831489Title: Fundamental Understanding and Performance Enhancement of Conductive Adhesive for Microelectronic Packaging Applications
Investigators: Wong, C. P.
Institution: Georgia Institute of Technology
EPA Project Officer: Bauer, Diana
Project Period: December 22, 2003 through December 21, 2008
Project Period Covered by this Report: December 22, 2003 through December 21, 2004
Project Amount: $325,000
RFA: Technology for a Sustainable Environment (2003)
Research Category: Pollution Prevention/Sustainable Development
Description:
Objective:The primary objectives of this research are to: (1) develop low-cost high performance electrically conductive adhesives (ECA) as a replacement of lead containing solders for environmentally friendly electronic packaging manufacturing; (2) understand corrosion behavior for ECA joints on various metal surfaces; (3) understand relationship between corrosion behavior and electrochemical potentials of ECA and various metal surfaces; (4) understand mechanism of corrosion control by cathode protection method in ECA joints and a role of sacrificial anode materials in the joints; (5) introduce reworkable and repairable resin binders to ECA formulation to reduce the waste of PCB or IC component to be produced during manufacturing; (6) introduce short chain aliphatic or conjugated organic compounds as well as properly functionalized carbon nanotubes into ECA formulations to increase the current carrying capability; (7) understand the thermomechanical failure of ECA joints, and improve the thermomechanical performance with conductive particles functionalized with self-assembled monolayer molecules; and (8) realize further energy savings by using variable frequency microwave curing of ECAs.
Progress Summary:During the first year of this research project, we tried many approaches to enhance the electrical properties of electrically conductive adhesives. Both the isotropically and anisotropically conductive adhesives ( ICA, ACA) were studied. Using the surface functionalization methodology, the conductive fillers were treated with self-assembled monolayer (SAM) and improved electrical properties such as bulk resistivity and contact resistance on non noble metals under harsh environments were observed. Reworkable ECAs for device repairing and flexible ECAs for thermocycling endurable properties were investigated.
Enhancement of Nano ECA Joint Resistance by Using Self-Assembled Monolayer
To improve the electrical properties of the anisotropically conductive adhesive (ACA) joints with fine pitch interconnect applications, two self-assembled monolayer (SAM) compounds were introduced to treat nano silver (Ag) fillers and compared (Kyoung-sik Moon and Wong, 2005).
Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), contact angle, and photoacoustic Fourier Transform Infrared (FTIR) results indicated the SAMs were well coated on the nano Ag particles. Furthermore, these SAM-treated ACAs were thermally stable at processing temperatures of the ACA samples.
By introducing the novel SAM materials into the interfaces between nano metal fillers and the substrate bond pads, the conductivity and current carrying capability of ACAs were improved significantly due to the stronger bonding between nano fillers and SAM and consequently the improved interface properties of the high performance ACA.
Different types of SAM compounds, dicarboxylic acid, and dithiol, were well coated on the nano Ag particles and formed strong bonding between nano Ag particles. The adherence of SAMs on nano Ag particles was thermally stable up to the curing temperature (150 ° C) of the ACA composites as a result of the larger surface area of nano particles and stronger bonding between SAM and nano fillers. With the introduction of SAM-treated nano Ag fillers and metal bond pads in ACAs, the electrical conductivity and current carrying capability of ACAs could be enhanced significantly as a result of the improved interfaces. For malonic acid treated nano ACA, the joint resistance could be achieved as low as 10-5 Ohm and the current carrying capability was higher than 3500mA. The improved electrical performance of SAM-treated nano Ag ACAs also was achieved with the increased thermal conductivity.
Improvement of Electrical Performance of Nano ECAs by Using Special Surfactants
Total contact resistance of ECA joints is a summation of contact resistance between the metal fillers, and between the fillers and the bond pad, and the bulk resistance of the fillers, which can be expressed in Equation (1).

For the ECAs to be used as the interconnect material in the ultra fine pitch applications (<20 μm), the size of current ECAs fillers (> 5 μm) is too large. Thus, the use of nano sized conductive fillers is being considered. For the nano fillers, however, the high contact resistance between the conductive fillers and its processibility has been an issue. As shown in Figure 1, the number of contacts between the fillers increases with the decrease of the filler size. This creates higher joint resistance when using the nano sized fillers. The fused interface in (c) would significantly reduce the filler-filler contact.
As shown in Figure 2, low temperature sintering behavior of nano metal particles has been observed recently ( Moon KS, Dong H, Maric R, Pothukuchi S, Li Y, Wong CP. Journal of Electronic Materials 2005;34(2):168-175). The nano Ag particles started to be sintered at 150 ° C.

Figure 1. Schematic Illustrations of ECAs Joints Incorporated With Large, Small and Sintered Fillers

Figure 2. Low Temperature Sintering of Ag Nanoparticles
The molecular dynamic simulation results on the low temperature sintering behavior of the nano Ag particles on an Au substrate was reported. (Dong, et al., 2004). This result is shown in Figure 3.
Another issue was to obtain the good dispersity of nanoparticles in polymer matrix. Thus, five different chain-length surfactants (S1-S5) were employed to improve the room temperature processibility. The low temperature sintering Ag nano particles were used as conductive fillers in ECA formulations. The thermal behavior of the surfactants on the nanoparticles was studied by using TGA and DSC, and the debonding temperatures and the debonding energy were determined. Finally, it was found that the length and the number of functional groups were critical factors that affect the dispersity and the sintering behavior during the epoxy curing process (Hongjin Jiang, et al., 2005).

Figure 3 . Molecular Dynamic (MD) Simulation of Ag Nanoparticles, (a) 400K and (b) 1000 K 2 nm Ag on Au Surface, Embedded Atom Method (EAM)
By using the surfactants, the room temperature viscosity was dramatically decreased and the mixing efficiency was significantly improved. Without the surfactants, 60 wt% was the maximum loading level to handle the mixture, whereas the filler loading was increased up to 70 wt% with the surfactants. From the bulk resistivity measurements, S3, S4, and S5 were effective in reducing the resistivity of the materials (see Figure 4 and Table 1). The order of 10-4 (cm) was obtained by nano particles of 70 wt%, although such a value can be shown in micron sized filler incorporated ECAs by 80 wt%.

Figure 4 . Bulk Resistivity of ECAs Incorporated With S1-S5 Treated Ag Nano Particles
The roles of the surfactants were to improve the processibility and the sintering efficiency during the epoxy curing process. The debonding of the surfactants from the particle before the full cure and the diffusion-out from the particles were desired for the well sintered morphology, because the remaining surfactants either on the particle or near the particles may hinder the particle-particle sintering process.
Table 1. Bulk Resistivity of ECAs Incorporated With S1-S5 Treated Ag Nano Particles (*The filler loading is 60 wt%; all the others are 70 wt%.)

Figure 5 shows the morphology of the cured nanocomposites (epoxy matrix) with S2 and S3 treated Ag, respectively. The S1 treated Ag formulation still exhibited many unsintered particles and less interconnection of the particle-particle interface; the S3 treated Ag showed significantly well sintered morphology and all the particle-particle interfaces were well interconnected. This may help the electron tunnel fluidity, resulting in the low bulk resistivity. By incorporating appropriate surfactants, the nanocomposites showed decent sintering behavior and considerably lower bulk resistivity.

Figure 5. Morphology of Cured Nanocomposites; (a) Nanocomposite Filled With Nano Ag/S2 and (b) Nanocomposite Filled With Nano Ag/S3
Development of Reworkable ECAs
Typical ECAs use epoxy resin as their matrix, which has superior properties over other polymers, such as high adhesion and low dielectric constant. Once cured, however, it is not reworkable. Reworkability is an important property of the interconnect materials, because the assembled IC chips should be easily repaired when the chip is incorrectly assembled. This enables the manufactures to save expensive high performance substrate boards.
In this study (Li and Wong, 2004), a liquid diepoxide was designed and synthesized, and used in isotropically conductive adhesive ( ICA) formulations. This diepoxide (see Figure 6) has a molecular structure that is able to thermally decompose at mild temperature, which allows selective individual removal of the failed components without damaging the board and its surroundings. The characterizations, including proton and carbon 13 nuclear magnetic resonance (NMR) infrared spectroscopy (IR), indicated the success of the synthesis.
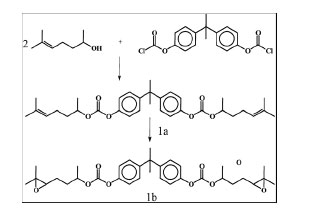
Figure 6 . Scheme of the Synthesis of the Diepoxide
A dual-epoxy system containing this secondary diepoxide and an equivalent bisphenol-A diepoxide were formulated and cured with an anhydride hardener and an imidazole catalyst (see Figure 7).

Figure 7. DSC Curing Diagram of the Dual-Epoxy System
Thermal analyses, such as differential scanning calorimetry, TGA, thermo-mechanical analysis (TMA, see Figure 8) and dynamic mechanical analysis (DMA, see Figure 9) were employed for the curing kinetics, thermal degradation behavior, glass transition temperature, coefficient of thermal expansion (CTE), and mechanical modulus, respectively.
The dual-epoxy system showed two exothermal curing peaks at 140 ° C and 180 ° C, respectively. The thermoset of this dual-epoxy system has a decomposition temperature around 234 ° C, a glass transition temperature around 80 ° to 90 ° C, and CTEs of 74 ppm/ ° C and 225 ppm/ ° C below and above its Tg, respectively.

Figure 8. TMA Diagrams of the Dual-Epoxy System and its Control

Figure 9. DMA Diagrams of the Dual-Epoxy System and its Control
The rework test on a surface mount component bonded to copper surface showed this ECA can be easily and quickly removed from the copper surface (see Figure 10).

Figure 10. Rework of Surface-Mounted Component. (a) A component mounted on a Cu laminated FR4 board. (b) Site board after component removal and immediate site cleaning. (c) Cleaned site
The bulk resistance and contact resistance of ICAs were measured before and during an accelerated aging process in a temperature/humidity chamber (85 ° C/85% RH). The ECA showed good bulk resistivity and contact resistance comparable to its control and commercial ECA gold and copper surface finishes (see Figures 11, 12, and 13).

Figure 11. Bulk Resistivity of an ECA Based on the Dual-Epoxy System, the Control, and Commercial ECA

Figure 12. Contact Resistance Change on Ni/Au Surface

Figure 13. Contact Resistance Change on Cu/ Organic Solderability Preservative (OSP) Surface
A new diepoxide containing a bisphenol-A moiety and two secondary carbonate linkages was designed, synthesized, and characterized with both NMR and FTIR spectroscopy. This diepoxide is a liquid at ambient temperature. A dual-epoxy system containing this secondary diepoxide and an equivalent bisphenol-A diepoxide were formulated and cured with an anhydride hardener and an imidazole catalyst. The curing properties of this diepoxide system were studied using DSC. Thermal properties of the cured resins of this diepoxide system were characterized with TGA, TMA, and DMA. The thermoset of this secondary diepoxide based dual-epoxy system showed a decomposition temperature around 240 ° C, a glass transition temperature around 80 ° C-90 ° C, and a CTE of 74 ppm/ ° C below its Tg. The rework test on a surface mount component bonded to a copper surface showed this ECA can be easily and quickly removed from the copper surface. The reliabilities of bulk resistivity and contact resistance of the ECA on nickel/gold, copper/OSP, and tin surfaces with respect to 85 ° C/85 percent RH aging were studied. The ECA showed good bulk resistivity and contact resistance that is comparable to its control and a commercial ECA on gold and copper finishes, but poor on the tin surface. In summary, the properties of the dual-epoxy system make it a promising reworkable ECA. The possible applications of this ECA could be rigid and flexible boards with Cu/OSP or Ni/Au surface finishes in a working temperature below 150 ° C.
Contact Resistance Stabilization
Electrochemical study of corrosion failure phenomena in ECA joints was carried out. To suppress the corrosion failure of the ECA joints, corrosion inhibitors and cathodic protection method were introduced to ECA formulations.
Incorporation of sacrificial anode materials . We have demonstrated that Zn as an additive was effective in stabilizing contact resistance of ECA joints on a non noble metal surface (Sn). We also found that particle size and loading level effect contact resistance. Cr, Al, and Mg were incorporated into ECA formulations and their effects on the contact resistance on SnPb, Sn, and SnAgCu were evaluated. Cr and Al were less effective than Zn and Mg, because Cr and Al have a more self-passivated nature. As shown in Figure 14, a certain amount of Mg could stabilize contact resistance of the joints on non noble metal pads significantly during the 85 ° C/85 percent RH test.

Figure 14. Contact Resistance of Mg Incorporated ECA Formulations on Various Surfaces
We measured corrosion potential values of metal pad surfaces such as Sn/Ag, Sn/Ag/Cu, Cu, and Sn/Pb, and sacrificial anode material incorporated ECAs. In this study, Al, Zn, Mg, and two Al alloys were used as sacrificial anode material additives. The mixtures of two types of metal powder and the alloys were incorporated into the ECA formulation. The difference in corrosion potential between metal pads and the ECAs is a primary driving force for corrosion occurrence in the joints, together with water molecules and ion impurity. Contact resistance of these ECAs on those metal pads was monitored and the results were compared with the potential differences that are shown in Table 2. We found that the contact resistance stability was closely related with the corrosion potential difference between the metal pads and the ECAs. Thus, we could confirm that galvanic corrosion is a primary driver of unstable contact resistance in the ECA joints.
Table 2. Potential Measurement and Resistance of Mixture and Alloys Incorporated ECA

Enhancement of Electrical Conductivity of ECAs by Functionalizing the Conductive Fillers . Short chain organic compounds were introduced into ECA formulations for improving the electrical properties. They will act as interfacial self-assembled monolayer modifiers for specific interactions with surface finishes on component lead and substrate pad.
Three short-chain difunctional carboxylic acids—acid M, acid A, and acid T—were used in a typical ECA formulation. The conductivity of ECA could be improved significantly by in situ removal or replacement of the C-18 stearic acid on Ag flakes for ECA formulations. The addition of only a small amount of acid M and acid A could increase the conductivity of ICA significantly. Acid M performed the best for the improvement of electrical conductivity of the ECA (35% improvement for conductivity). The connection of silver flake with short-chain dicarboxylic acid facilitates electron transport along the chain and thereby improves the electrical conductivity.
Contact Resistance Stabilization With Suitable Corrosion Inhibitors . The contact R on Cu surfaces for all samples were relatively stable because the Cu surfaces used have been treated with OSP. The contact resistance of a control ECA on a Sn surface increased dramatically with aging. With the addition of different types of additives, the contact resistance was dramatically stabilized, especially by A1 or A4. The contact resistance change of A1 added ECA was less than 40 percent while ECA with A4 showed an even better result after over 600 hours of aging. Therefore, the two additives, A1 and A4 were very effective in stabilizing contact resistance of the ECA joints and it is likely that the additives play a role of the corrosion inhibitor for Sn surfaces. This result is published in Li, et al., 2005.
Enhancement of Thermomechanical Properties via Flexible Matrix Materials
Thermomechanical failure of ECA joints was studied. To improve the thermomechanical performance of ECA materials, the polymer matrix material was modified with flexible molecules. Bis-F type epoxy, rubber modified epoxy, and in-house synthesized (rubberized) epoxies were blended by various ratios. Their physical, mechanical, and thermal properties were characterized. In addition, the ECAs were fabricated with these polymer matrices and the 1206 resistors were assembled on the test boards with the ECAs. The electrical resistance of the chip and board joints was monitored under the air-to-air thermal cycling (AATC, -55 ° C to 125 ° C) test. We found that the elastic modulus is an important factor for high TC performance. For TC performance, it was found that the Tg should be higher than the upper limit of testing temperature, because the modulus and other material properties are dramatically decreased above the Tg. Low moisture uptake and high adhesion yield also were important parameters. In addition, interfacial crack and delamination were found in TC failed samples as shown in Figure 15.

Figure 15. Joint Interfaces After AAT; (a) Non Failed Sample on Sn Surface and (b) Failed Sample on Cu/OSP Surfaces
Journal Articles on this Report : 5 Displayed | Download in RIS Format
| Other project views: | All 75 publications | 31 publications in selected types | All 30 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Dong H, Moon KS, Wong CP. Molecular dynamics study on the coalescence of Cu nanoparticles and their deposition on the Cu substrate. Journal of Electronic Materials 2004;33(11):1326-1330 |
R831489 (2004) |
not available |
|
|
Dong H, Zhang ZQ, Wong CP. Molecular dynamics study of a nano-particle joint for potential lead-free anisotropic conductive adhesives applications. Journal of Adhesion Science and Technology 2005;19(2):87-94 |
R831489 (2004) R831489 (2005) |
not available |
|
|
Dong H, Moon KS, Wong CP. Molecular dynamics study of nanosilver particles for low-temperature lead-free interconnect applications. Journal of Electronic Materials 2005;34(1):40-45 |
R831489 (2004) R831489 (2005) |
not available |
|
|
Jiang HJ, Moon KS, Zhang ZQ, Pothukuchi S, Wong CP. Variable frequency microwave synthesis of silver nanoparticles. Journal of Nanoparticle Research 2006;8(1):117-124 |
R831489 (2004) R831489 (2005) |
not available |
|
|
Moon KS, Li Y, Xu JW, Wong CP. Lead-free interconnect technique by using variable frequency microwave. Journal of Electronic Materials 2005;34(7):1081-1088 |
R831489 (2004) R831489 (2005) |
not available |
electrically conducive adhesives, anisotropically conductive adhesive, self-assembled monolayer thermogravimetric analysis, differential scanning calorimetry, Fourier transform infrared spectroscopy, FTIR, isotropically adhesive, surfactants, cured nanocomposites, nanoparticles, dual-epoxy system, contact resistance, flexible matrix materials,
,
INTERNATIONAL COOPERATION, TREATMENT/CONTROL, Sustainable Industry/Business, Scientific Discipline, RFA, POLLUTION PREVENTION, Technology for Sustainable Environment, Sustainable Environment, Chemical Engineering, Technology, Energy, Environmental Chemistry, Ecology and Ecosystems, Economics and Business, energy conservation, cleaner production, waste reduction, clean technologies, lead reduction, environmentally benign adhesive, environmentally conscious manufacturing, energy efficiency, electronic packaging, corrosion resistant
Progress and Final Reports:
Original Abstract
2005 Progress Report
2006 Progress Report
2007 Progress Report
Final Report
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20090825061943im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)