|
Publications
> Informational Brochures
> Working to Prevent the Diversion and Abuse of OxyContin


Background
In recent months, concern has grown among federal,
state, and local officials about the dramatic increase in the illicit
availability and abuse of the prescription drug OxyContin®
(The controlled release form of oxycodone)
In response, the Drug Enforcement Administration
(DEA) initiated a comprehensive effort in February 2001 to prevent the
diversion of OxyContin® and reverse this trend.
OxyContin® is legitimately used to treat moderate
to severe pain. It is fast becoming the drug of choice for pain management
with sales reaching more than $ one billion.
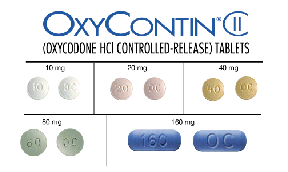
OxyContin® is available in 10, 20, 40, 80, and 160
milligram doses.
The Problem
Abusers can easily compromise the controlled release
formulation for a powerful morphine-like high.
Common means of OxyContin® diversion
- Fraudulent prescriptions
- Doctor shopping
- Over-prescribing
- Pharmacy theft
- Organized rings of individuals diverting and
selling OxyContin®
- Foreign diversion and smuggling into the U.S.
While the diversion and abuse of OxyContin® appear
to be concentrated in rural areas of the eastern United States, there are
growing problems throughout the nation.
Criminal activities resulting from the abuse of
OxyContin® are challenging local law enforcement and health care system
resources. Property and other crimes related to OxyContin® addiction have
been reported to have increased as much as 75 percent.
DEA’s Goal
To ensure that the legitimate users of OxyContin®
continue to receive their medication while reducing its diversion and
abuse
Enforcement & Intelligence
Focus existing resources on investigations of
illicit sales and abuse of OxyContin®
- Investigate pharmacy thefts with other law
enforcement agencies
- Identify large volume purchasers
- Participate in Healthcare Fraud Working Groups
- Work with international organizations
Regulatory & Administrative
- Utilize the full range of DEA’s authority to
restrict abuser access to OxyContin®
- Work closely with the FDA urging reformulation to
reduce abuse potential
Seek Industry Cooperation
- Encourage Purdue Pharma to develop a balanced
market strategy
- Solicit support in providing educational programs
Outreach
- Increase awareness among:
- The Healthcare Industry
- Other Government Agencies
- The General Public
- Seek the medical community’s input on ways to
lessen and prevent diversion of OxyContin®
- Meet with major private and government insurance
carriers
- Develop resources for use by other agencies in
alerting practitioners to the problem
For more information:
Please contact your nearest DEA
office
Or
Visit one of our Internet Web Sites:
www.DEAdiversion.usdoj.gov
Or
www.dea.gov
Presented as a public service by:
Drug Enforcement Administration
Office of Diversion Control
Washington, D.C. 20537

Presented as a public
service by:
Drug Enforcement
Administration
Office of Diversion Control
Washington, D.C. 20537
Download
PDF for Printing
Download Adobe
Acrobat Reader to view and print PDF files
Back to
Top
Registration
Support
Toll Free Number: 1-800-882-9539
ARCOS
| Career Opportunities | Chemical Program |
Controlled
Substance Schedules | Drugs and
Chemicals of Concern
Electronic Commerce Initiatives | | Federal Register
Notices | Import Export | Links
| Meetings
and Events | NFLIS
Offices &
Directories | On-Line Forms & Applications |
Program
Description | Publications
|
Questions & Answers | Quotas
Reports Required by 21 CFR | Title 21 Regulations & Codified
CSA
Contact Us | Home
| Hot
Items | Site Map | Search | What's New
|