
| |
| |
Content Reviewed 09/25/2008
|
|
 |
Hospital eTool
Surgical Suite Module
|
 |
|
Click on the area for more specific
information.
|
Common safety and health topics:
Virtual Tour
Review the hazards below and then tour the virtual reality room.
|
The anesthetic gas and vapors that leak out into the surrounding room during medical
and surgical procedures are considered waste anesthetic gases.
They include nitrous oxide and halogenated agents (vapors) such as:
- Enflurane
- Isoflurane
- Sevoflurane
- Desflurane
- Halothane
Potential adverse health effects of exposure to waste
anesthetic gases include loss of consciousness, nausea, dizziness, headaches, fatigue,
irritability, drowsiness, problems with coordination and judgment, as well
as sterility, miscarriages, birth defects, cancer, and liver and kidney
disease.
Potential Hazard
-
Exposure to waste anesthetic gases occurs from:
- Poor work practices during the anesthetization of patients.
- Leaking or poor gas-line connections.
- Improper or inadequate maintenance of the machine.
- Patient exhalation in the recovery room or Post Anesthesia Care Unit (PACU) during off-gassing of surgery patients.
Possible Solutions
OSHA's
Guidelines for Workplace Exposures
to Anesthetic Gases provide the following recommendations provides the following
recommendations:
- Use appropriate anesthetic gas scavenging systems in
operating rooms.
- Appropriate waste gas evacuation
involves collecting and removing waste gases, detecting and correcting leaks, considering work practices, and effectively ventilating
the room (Dorsch and Dorsch 1994).
- Provide enough ventilation in the
surgical suite to keep the room
concentration of waste anesthetic gases below
the applicable occupational exposure levels.
The ventilation design and specifications
should meet the most current American
Institute of Architect’s Guidelines for
Design and Construction of Health Care
Facilities.
- To minimize waste anesthetic gas concentrations in the operating room, the recommended air exchange rate (room dilution ventilation)
is a minimum total of 15 air changes per hour with a minimum of 3 air changes of outdoor air (fresh air) per hour (American Institute of
Architects 2006).
- Use a properly designed and operating dilution ventilation system to minimize waste anesthetic
gas concentrations in recovery room areas.
- System should provide a recommended minimum total of 6 air changes per hour with a minimum of 2 air changes of outdoor air per
hour (American Institute of Architects
2006).
- Conduct periodic exposure monitoring with
particular emphasis on peak gas levels
in the breathing zone of nursing
personnel working in the immediate
vicinity of the patient's head.
- Note: Methods using random room sampling
to assess ambient concentrations of
waste anesthetic gases in the recovery
room are not an accurate indicator of
the level of exposure experienced by
nurses providing bedside care. Due to the closeness of the recovery room
nurse to the patient, such methods would
consistently underestimate the level of
waste anesthetic gases in the breathing
zone of the bedside nurse. Therefore,
personal sampling is required to
determine the employee's overall
workplace exposure to waste anesthetic
gases.
- Implement a routine ventilation system maintenance program to keep waste
anesthetic gas exposure levels to a minimum.
In addition, the
Hospital Investigations: Health Hazards Chapter of the OSHA Technical
Manual recommends that:
- Vaporizers of anesthesia machines be
turned off when not in use. Proper face
masks, sufficiently inflated endotracheal
tubes, and the prevention of anesthetic
spills will decrease the amount of waste
anesthetic gases in the operating room.
- Inspection and maintenance of anesthesia
machines be conducted by factory service
representatives or other qualified personnel
at least every four months. Leakage of gas
should be less than 100 ml/min during normal
operation. During normal operation, employee
exposure to anesthetic gases in use should
not exceed the NIOSH recommended exposure
limits.
- Prior to each day's use, a complete
check of all anesthesia equipment
(connectors, tubing, etc.) be conducted.
- Spills of liquid anesthetic agents be
cleaned up promptly.
- Information be provided and a training
program implemented in accordance with
OSHA's Hazard Communication Standard [29
CFR 1910.1200] for all employees exposed
to waste anesthetic gases. See
Healthcare-wide Hazards -
Hazardous Chemicals.
Additional Information:
- Anesthetic Gases:
Guidelines for Workplace Exposures. OSHA,
(2000, May 18). Provides guidelines and controls to help reduce occupational exposure to waste anesthetic gases.
- Waste Anesthetic Gases.
OSHA
Safety and Health Topics Page.
- U.S. Department of Health and Human
Services (DHHS), National Institute
for
Occupational Safety and Health (NIOSH)
-
Guidelines for design and
construction of health care facilities. American Institute of Architects,
Academy of Architecture for Health, (2006).
-
Recommended practices for a safe environment of
care.
Association of Perioperative Registered
Nurses (AORN), In: Perioperative Standards
and Recommended Practices, (2008):351-374.
-
Waste Anesthetic Gases: Information for
Management in Anesthetizing Areas and the
Postanesthesia Care Unit (PACU). American
Society of Anesthesiologists, (2004), 2 MB
PDF,
45 pages.
|
Potential Hazard
-
Occupational exposure to blood and other
potentially infectious materials (OPIM)
places employees at risk of infection from
bloodborne pathogens such as Hepatitis B
Virus (HBV), Hepatitis C Virus (HCV) and
Human Immunodeficiency Virus (HIV) while
performing surgery-related tasks.
Possible Solutions
Among other things, OSHA’s
Bloodborne Pathogens Standard requires that:
- Engineering and work practice controls
be used to eliminate or minimize exposures
to blood and OPIM.
[29
CFR 1910.1030(c),
29 CFR 1910.1030(d), and
OSHA Directive CPL CPL 02-02-069]
In addition, sharps injuries in the surgical
area must be eliminated or minimized through use of measures such as:
- Safer needle/other sharps devices.
- Blunt-tip suture needles.
- Needleless IV connectors.
- Proper containers for sharps.
- "No Pass Zone" for surgical instruments.
- Method for passing equipment safely between surgeon and assistants.
- The hands-free technique is a work practice whereby a tray or other means are used to
eliminate simultaneous handling of sharp instruments during surgery.
- Appropriate personal protective
equipment (PPE) be worn if blood or OPIM exposure is
anticipated. [29
CFR 1910.1030(d)(3)] The PPE must be
impermeable under normal conditions of use
and for the duration of time it will be
used. The type of PPE
depends on the anticipated exposure.
Appropriate PPE includes, but is not limited
to, gloves, gowns, face shields or masks,
and shoe covers. For example:
- Gloves must be worn when hand contact with blood, mucous membranes, OPIM, or non-intact skin is anticipated, or when handling
contaminated items or surfaces
[29
CFR 1910.1030(d)(3)(ix)].
- Masks, in combination with eye
protection devices, must be worn whenever
splashes, spray, splatter or droplets of
blood or OPIM may be generated. [29
CFR 1910.1030(d)(3)(x)]
- Contaminated needles and other
contaminated sharps be discarded immediately or as soon as feasible into appropriate containers.
[29
CFR 1910.1030(d)(4)(iii)(A)(1)]
- Sharps containers be located as close as is feasible to the
immediate where sharps are used or reasonably anticipated to be found.
[29
CFR 1910.1030(d)(4)(iii)(A)(2)(i)].
- Contaminated needles and other contaminated sharps must not be bent, recapped, or removed except as noted in paragraphs
(d)(2)(vii)(A) and
(d)(2)(vii)(B). Shearing or breaking contaminated needles is
prohibited.
- Employers ensure that handwashing facilities be readily
accessible,
[29
CFR 1910.1030(d)(2)(iii)]
and that employees wash their hands immediately or as soon as feasible after removal of gloves
or other personal protective equipment
[29
CFR 1910.1030(d)(2)(v)].
- Hand must be washed with an appropriate
soap and water, whenever there has been
occupational exposure to blood or OPIM. If a
sink is not readily accessible (e.g., in the
field) for instances where there has been
occupational exposure, hands may be
decontaminated with hand cleanser or
towelette, but must be washed with soap and
running water as soon as feasible.
- If there has been no occupational
exposure to blood or OPIM, use of an
appropriate antiseptic hand cleanser is
acceptable.
Additional Information:
-
Acceptable use of antiseptic-hand cleansers
for bloodborne pathogen decontamination and
as an appropriate handwashing
practice [1910.1030; 1910.1030(d)(2)(v);
1910.1030(d)(2)(vi)]. OSHA Standard
Interpretation, (2003, March 31).
-
Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to
Surgical Personnel. OSHA and the National Institute for Occupational
Safety and Health (NIOSH) Publication No. 2008-101, (2007, October). Also
available as a 281 KB
PDF, 4 pages. Supersedes NIOSH Publication 2007–132.
-
Guideline for Hand
Hygiene in Health-Care Settings.
Centers for Disease Control and Prevention
(CDC), Morbidity and Mortality Weekly Report
(MMWR) 51(RR16), (2002, October 15), 494 KB
PDF, 56 pages.
-
Workbook for Designing, Implementing, and
Evaluating a Sharps Injury Prevention
Program. Centers for Disease Control and
Prevention (CDC).
-
Bloodborne Infectious Diseases HIV/AIDS,
Hepatitis B Virus, and Hepatitis C Virus:
Preventing Needlesticks and Sharps Injuries. NIOSH
Safety and Health Topic.
- Association
of Perioperative Registered Nurses (AORN)
-
B. Stringer, C. Infante-Rivard, J.A. Hanley.
Effectiveness of the hands-free techniques
in reducing operating theatre injuries.
Occupational and Environmental Medicine.
2002, October;Volume (59, No. 10):703.
-
B. Stringer, T. Haines. Hands-free
technique: preventing occupational exposure
during surgery. Journal of
Perioperative
Practice. 2006, October;Volume (16, No. 10):
495.
-
Virginia Health System.
The University of Virginia.
 For additional information, see
Healthcare-wide Hazards -
Bloodborne Pathogens, and
Needlesticks.
For additional information, see
Healthcare-wide Hazards -
Bloodborne Pathogens, and
Needlesticks.
|
Potential Hazard
-
Developing latex allergy from exposure to products that contain latex such as gloves, catheters, and tubing.
Possible Solutions
- Provide appropriate gloves, including
powderless, hypoallergenic, glove liners, or other similar alternatives to employees who are allergic to the gloves normally provided
[29
CFR 1910.1030(d)(3)(iii)].
Note: Do not
assume hypoallergenic gloves are non-latex or latex-free.
In addition, good work
practices should be used. These may include:
- Providing a latex-safe work environment.
- Using non-latex gloves and other latex-free products.
- Selecting a low protein, powder-free glove.
Additional Information:
 For additional information, see
Healthcare-wide Hazards -
Latex Allergy.
For additional information, see
Healthcare-wide Hazards -
Latex Allergy.
|
Within a healthcare
facility, compressed gases are usually
either fixed piped gas systems
or individual cylinders of
gases.
Potential Hazard
-
Potential hazards associated with compressed
gas will vary based on the chemicals;
however, they may include fire, explosion,
and toxicity.
|
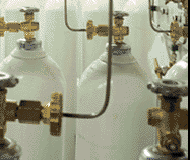
Figure 1. Cylinders of compressed gas. |
Possible Solutions
-
Store, handle, and use compressed gases in
accordance with
29 CFR
1910.101 and Pamphlet P-1-1965 from the
Compressed Gas Association.
-
All cylinders whether empty or full must be
stored upright.
-
Secure cylinders of compressed gases.
Cylinders should never be dropped or allowed
to strike each other with force.
-
Transport compressed gas cylinders with
protective caps in place and do not roll or
drag the cylinders.
|

Figure 2. Caution: Keep
All Cylinders
Chained. |
Additional Information:
- 1910.101, Compressed
gases.
OSHA Standard.
- 1910.103, Hydrogen.
OSHA Standard.
- 1910.104, Oxygen.
OSHA Standard.
- 1910.105, Nitrous oxide.
OSHA Standard.
-
Compressed Gas and Equipment. OSHA
Safety and Health Topics Page.
-
Standard for Health Care Facilities, Chapter
4, Gas and Vacuum Systems. National Fire
Protection Association, NFPA 99, (2005).
|
Medical staff in a surgical setting often assume
prolonged awkward postures. Typically, employees vary in height which may
require work surfaces at differing heights to minimize awkward postures.
Potential Hazards
- Standing in static postures
continuously during lengthy surgical
procedures, causes muscle fatigue and pooling of blood in the lower extremities.
- Standing on hard work surfaces such as
concrete creates trauma and pain to the feet.
- Awkward postures
resulting from prolonged standing, trunk
flexion, neck flexion, and arms held
higher than the optimal working height.
Possible Solutions
- Provide stools, where possible.
- Use shoes with well-cushioned insteps and soles.
- Provide a footrest bar or a low stool, allowing employees
to continually alter their posture by raising one foot.
- Use height-adjustable work tables and surfaces.
- Use anti-fatigue mats.
Additional Information:
-
Association
of Perioperative Registered Nurses (AORN)
-
Ergonomically Healthy Workplace Practices. (2006, March).
-
Guidance Statement: Safe Patient Handling and Movement
in the Perioperative Setting.
(2007).
-
Recommended practices for positioning the patient in the
Perioperative
practice setting.
In: Perioperative Standards and Recommended Practices, (2008):497-520.
 For additional information, see
Healthcare-wide Hazards -
Ergonomics, Awkward Postures.
For additional information, see
Healthcare-wide Hazards -
Ergonomics, Awkward Postures.
|
Laser or electrosurgical units may be required
during surgical procedures. Smoke byproduct or
"plume" is created when tissue is thermally
destroyed. Smoke plume may contain toxic gases
and vapors such as benzene, hydrogen cyanide,
and formaldehyde, bioaerosols, dead and live
cellular material (including blood fragments),
and viruses.
The research is limited on transmission of
disease through surgical smoke, but the
potential for generating infectious viral
fragments, particularly during treatment of
venereal warts, may exist. Researchers have
suggested that the smoke may act as a vector for
cancerous cells which may be inhaled by the
surgical team and other exposed individuals.
Potential Hazards
-
Exposure to high concentrations of smoke may
cause ocular and upper respiratory tract
irritation and create visual problems for the
perioperative team.
-
Smoke may contain toxic gases that could have
the potential for adverse health impacts, such
as mutagenic and carcinogenic impacts.
Possible Solutions
- Use portable smoke evacuators and room suction systems
with inline filters.
- Keep the smoke evacuator or room suction hose nozzle inlet within 2 inches of the surgical site to effectively capture
airborne contaminants.
- Have a smoke evacuator available for
every operating room where plume is
generated.
- Evacuate all smoke, no matter how much
is generated.
- Keep smoke evacuator "ON" (activated) at all times when airborne particles are produced during all surgical or other
procedures.
- Consider all tubing, filters, and absorbers as infectious waste and dispose of them appropriately. Use
Universal Precautions as required by
the OSHA Bloodborne Pathogens Standard when
contaminated with blood or OPIM [29
CFR 1910.1030(d)(1)].
- Use new tubing before each procedure
and replace the smoke evacuator filter as recommended by the manufacturer.
- Inspect smoke evacuator systems regularly to
ensure proper functioning.
Additional Information:
-
Laser/Electrosurgery Plume.
OSHA
Safety and Health Topics Page.
-
Control of Smoke from Laser/Electric
Surgical Procedures. U.S. Department of
Health and Human Services (DHHS), National
Institute for
Occupational Safety and Health (NIOSH) Publication No. 96-128,
(1998, March 2).
-
Association of Perioperative Registered
Nurses (AORN)
-
Position Statement: Surgical Smoke and
Bio-Aerosols
-
Recommended practices for a safe environment
of care. In: Perioperative
Standards and Recommended Practices,
(2008):351-374.
-
Recommended practices for electrosurgery. In:
Perioperative Standards and
Recommended Practices, (2008):315-329.
-
Recommended practices for endoscopic
minimally invasive surgery. In:
Perioperative Standards and Recommended
Practices, (2008):331-343.
|
Although there are hundreds of different
types of lasers, only about a dozen laser
systems are found in everyday clinical
use. Nearly all laser products used in
surgery are
Class 4 as they are
designed to deliver laser radiation for
the purpose of altering biological tissue.
When lasers are
introduced into a healthcare environment,
professionals must be prepared to address
safety issues for both the staff and
patient. Safe use of these systems
requires an understanding of the engineering, training, and administrative
requirements for all elements of a healthcare system as well as the risks
associated with use of laser light.
All medical lasers are
regulated and federal
regulations require manufacturers to
classify the medical laser system based
primarily on its ability to cause damage
to the eye and skin. This classification
must be indicated on the laser system’s
label ranging from Class 1 (no hazard) to
Class 4 (serious hazard).
For a more detailed discussion on lasers,
see the
Use of Medical Lasers.
Potential Hazard
Possible Solutions
The American National Standard Institute (ANSI)
Z136 series of laser safety standards covers lasers in
medical settings and provides guidance for the
safe use of lasers for diagnostic, cosmetic,
preventative and therapeutic applications in
healthcare facilities. These guidelines are
considered to be the standard for safe practice
in the industry and include solutions such as:
- Use laser protective
eyewear that provides adequate protection
against the specific laser wavelengths
being used. All laser
eyewear must be marked with Optical Density (OD) and laser
wavelength.
- Display warning signs conspicuously
on all doors entering the Laser
Treatment Controlled Area (LTCA), so as to
warn those entering the area of laser use.
Warning signs should be covered or removed
when the laser is not in use.
- Maintenance on lasers and laser systems
must be performed only by facility-authorized
technicians trained in laser service.
- Provide local exhaust ventilation with a
smoke evacuator or a suction system with an
in-line filter to reduce
laser-generated airborne
contaminants (LGAC) levels in laser
applications.
- Use an appropriate filter or barrier which reduces
any transmitted laser radiation to levels
below the applicable Maximum Permissible
Exposure (MPE) level, for all facility windows (exterior or interior) or
entryways located within the Nominal
Hazard Zone (NHZ) of a
Class 3B and
Class 4
laser system.
|

Figure 3.
Goggles.

Figure 4. Class 2 Laser Sign
stating: "Caution. Laser Radiation. Do not
stare into beam."

Figure 5.
Class 4 Laser Sign stating: "Danger.
Laser Radiation. Avoid eye or skin exposure
to direct or scattered radiation." |
- Ensure that alignment and calibration
techniques are used for routine Perioperative
checkout of the laser system.
- Use skin protection if repeated exposures
are anticipated at exposure levels at or near
the applicable MPE limits for the skin.
- Provide detailed
training in laser safety
for healthcare personnel using or working in
the presence of Class 3B and Class 4 healthcare laser systems.
- Ensure
credentialing of staff using laser
systems.
|
Additional Information:
- 1926.54, Nonionizing radiation.
OSHA Standard.
-
Laser Hazards.
OSHA
Safety and Health Topics Page.
-
OSHA Technical Manual (OTM). OSHA
Directive TED 01-00-015 [TED 1-0.15A],
(1999, January 20).
- Laser Hazards.
Contains information that will assist in the recognition and
evaluation of laser hazards.
-
U.S. Department of Health and Human
Services, Food and Drug Administration
(FDA), Center for Devices and Radiological
Health (CDRH)
- International Electrotechnical
Commission
- IEC 60825-1/A2:2001. Safety of Laser
Products - Part 1: Equipment classification,
requirements, and user’s guide.
- IEC 60825-2 IS 01. Interpretation Sheet
1
- Laser Institute of America (LIA). The
LIA is the
secretariat and publisher of the ANSI Z136
series of laser safety standards. They are
recognized as a minimum standard for laser
safety.
- ANSI Z136.1-2007. American
National Standard for the Safe Use of
Lasers.
- ANSI Z136.3-2005. American National Standard for the Safe Use of Lasers
in Health Care Facilities.
- Recommended practices for laser safety
in practice setting. Association of
Perioperative Registered
Nurses (AORN), In: Perioperative Standards and Recommended
Practices, (2008):447-452.
|
|
Potential Hazard
-
Exposure to possible hazardous chemicals found and used in the surgical area
typically during mixing, preparation, and in
the operating room.
-
This may include peracetic acid used in cold
sterilant machines, Methyl Methacrylate (MMA),
an acrylic cement-like substance used to
secure prostheses to bone during orthopedic
surgery, and
waste anesthetic gases.
Possible Solutions
- Mix methyl methacrylate only in a closed system.
- Carefully read and follow instructions and warnings on labels, (e.g., when using cold sterilant machines for
sterilizating equipment that cannot be autoclaved, use goggles provided and do not open machine until it is in a
safe to open mode).
- Consider using disinfectants or other products that are not hazardous.
- Inform employees of chemical hazards and have on hand Material Safety
Data Sheets, (MSDS) for all hazardous chemicals used in their facilities.
[29 CFR 1910.1200]
- Follow all MSDS instructions regarding safe handling, storage, and disposal of hazardous chemicals.
 For additional information, see
Healthcare-wide Hazards -
Hazardous Chemicals
and
Glutaraldehyde.
See also Central Supply –
Ethylene Oxide.
For additional information, see
Healthcare-wide Hazards -
Hazardous Chemicals
and
Glutaraldehyde.
See also Central Supply –
Ethylene Oxide. |
Potential Hazard
-
Exposure to burns or shocks from poorly
maintained equipment (e.g., autoclaves,
warming cabinets, defibrillators) or
improperly trained staff.
Possible Solutions
-
Create a safety and health program that
routinely monitors the condition of equipment
and addresses work practices of employees.
This program should include practices such as:
- Train employees to correctly and safely use
and clean equipment.
- Maintain adequate working space and access to equipment.
- Visually inspect equipment before using.
- Visually inspect cords. Do not
use if frayed or damaged.
- If something does not look right, do not use the machine and call for assistance.
- Ensure that all electrical service
equipment near sources of water are properly grounded
[29
CFR 1910.304].
- Use appropriate personal protective equipment and safe work practices for assessed hazards (e.g., when handling hot items
use gloves, and do not open autoclaves or sterilizers until items are sufficiently cooled).
- Adhere to all manufacturer and
operator instructions to ensure safe use
of equipment.
Additional Information:
 For additional information, see
Healthcare-wide Hazards -
Electrical, and
PPE.
For additional information, see
Healthcare-wide Hazards -
Electrical, and
PPE.
|
Potential Hazards
-
Falling over portable equipment of a color
that visually
blends into the floor.
-
Slipping on debris (bandages, tubing, blood,
IV fluids) that had fallen or spilled on the
floor.
- Tripping on
electrical cords that may cross floors.
Possible Solutions
- Keep aisles and passageways clear and in good
repair, with no obstructions across or in aisles
that may create a
hazard [29
CFR 1910.22(b)(1)].
- Provide ceiling or floor outlets for equipment to
ensure that power cords do not run across pathways.
- Mark mobile equipment (e.g., stools) with a bright color, or a taped "X",
making them more visible and distinguishable from the floor.
Tape should be washable and durable.
 For additional information, see
Healthcare-wide Hazards -
Slips/Trips/Falls
For additional information, see
Healthcare-wide Hazards -
Slips/Trips/Falls
|
|
Accessibility Assistance: Contact the OSHA
Directorate of Technical Support and Emergency Management at 202-693-2300 for
assistance accessing PDF materials.
|
