| |
     |
< Previous | Home | Next > Chapter
9
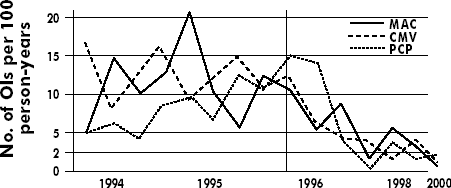
Overview TOP What are the major opportunistic diseases (ODs) in patients with HIV/AIDS? The diseases that occur as a result of HIV-related immunodeficiency include both opportunistic infections (OIs) and malignancies. The risk of developing an OI has declined dramatically with the widespread use of potent combination antiretroviral therapy (ART) (see Figure 9-1). The major OIs that occur in patients with HIV are Pneumocystis carinii pneumonia (the organism that causes human disease in PCP is now classified as Pneumocystis jiroveci, but PCP remains the conventional abbreviation in clinical use), tuberculosis, disseminated Mycobacterium avium complex (MAC) disease, cytomegalovirus (CMV) disease, Candida esophagitis, central nervous system (CNS) infections such as cryptococcal meningitis or Toxoplasma encephalitis, and cryptosporidiosis. While Mycobacterium tuberculosis is an infection that occurs in both immunocompetent and immunosuppressed patients, it remains one of the most common opportunistic co-infections in persons with HIV disease and is responsible for considerable morbidity and mortality worldwide. A host of other OIs may be seen less commonly or in patients in geographic areas where specific infections are endemic, eg, histoplasmosis in the central Midwest part of the U.S. or isosporiasis in South Florida and Puerto Rico. Figure
9-1. Decline in the Incidence of Opportunistic Infections in HIV-1-Infected
Individuals Receiving Potent Combination Antiretroviral Therapy Source: Adapted from Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-860. Copyright 1998 by the Massachusetts Medical Society. Modified with permission (data extrapolated through 2000). What factors are associated with higher risk of developing an OI? OIs occur primarily in HIV-infected individuals who are not receiving either OI prophylaxis or ART (or who have not responded to it with an increase in their CD4 cell counts above the threshold that predicts risk of developing an OI). The principal risk of developing a specific OI is determined by the degree of immunosuppression, as measured by the CD4 cell count. The CD4 threshold of risk differs for each specific OI (see Table 9-1). It is uncommon for an OI to occur in HIV-infected individuals with CD4 cell counts above its threshold. For patients with the same CD4 cell count, those with high viral loads (plasma HIV RNA levels >100,000 copies/mL) have a greater risk for developing an OD than those with a low viral load. Other factors that contribute to increased risk for ODs include previous exposure to or infection with a specific pathogen, prior occurrence of an OD, environmental exposure in the absence of a host response, and the use of ART. What malignancies are associated with HIV infection? The most common are Kaposi sarcoma (KS) and lymphomas. There is also a modest increase in the frequency of cervical cancer. In general, these 3 forms of malignancies correlate with immunosuppression, meaning the frequency increases with low CD4 cell counts, but the association is less strong than it is with the traditional AIDS-defining ODs. The rate of KS is approximately 20,000-fold higher with HIV infection compared with the general population, the frequency of non-Hodgkin lymphoma is 200- to 600-fold more frequent with HIV infection, and the rate of cervical cancer is 5-fold greater. Both lymphomas and KS are less frequent in the era of ART. Pneumocystis Jiroveci (Carinii) Pneumonia (PCP) TOP What are the clinical manifestations of PCP in patients with HIV infection? PCP usually presents as an acute or subacute respiratory illness associated with fever, dyspnea, nonproductive cough, and fatigue. Physical findings on examination include tachypnea, fever, and inspiratory rales. Rarely, disseminated Pneumocystis occurs in HIV-infected patients with profound immunosuppression. Laboratory abnormalities associated with PCP include leukocytosis, hypoxemia, an elevated lactate dehydrogenase (LDH), and chest radiographic findings of localized or diffuse interstitial infiltrates. Occasionally, nodular or cavitary lesions may be observed. Pneumothorax in patients with advanced HIV is almost always associated with underlying Pneumocystis infection. The degree of hypoxemia is a measure of disease severity; severe PCP is defined as an arterial blood gas PO2 of <70 mm Hg with an arterial-alveolar O2 gradient (AaO2) of >35 mm Hg. The diagnosis is confirmed by demonstrating the presence of P. jiroveci organisms in sputum or tissue samples obtained by sputum induction, bronchoscopy with bronchoalveolar lavage, or tissue biopsy. In the rare circumstance in which acute PCP presents very early with a normal chest radiograph, a gallium scan may demonstrate diffuse uptake in the lung compatible with an interstitial inflammatory process; however, this diagnostic test is nonspecific. How should you manage PCP? The preferred initial treatment for PCP in HIV-infected individuals is trimethoprim-sulfamethoxazole (TMP-SMX). Route of administration (oral or parenteral) is generally based on the severity of the disease. For those with severe PCP, parenteral therapy with trimethoprim 15-20 mg/kg and sulfamethoxazole 75-100 mg/kg, in 6-8 hour divided doses, is recommended. In addition, adjunctive treatment with corticosteroids, in a dose equivalent to 40 mg twice a day of prednisone for the first 5 days, followed by a tapering schedule of 40 mg/day for days 6-10, and then 20 mg/day to complete 21 days of therapy, should be initiated as soon as possible after starting treatment but preferably within the first 72 hours. For patients with mild to moderate PCP (PO2 >70 mmHg and AaO2 gradient <35 mmHg), oral treatment with 2 double-strength tablets of TMP-SMX every 8 hours is recommended. Gradual dose escalation may reduce the rate of occurrence of adverse reactions to TMP-SMX. For those unable to tolerate TMP-SMX, a number of alternative regimens are available for treatment (see Table 9-2).
What adverse reactions occur with treatment for PCP? Patients should be carefully monitored for adverse reactions to treatment and response to therapy. Many patients will demonstrate acute worsening of signs and symptoms and have radiographic abnormalities in the first 3-5 days of treatment. This is the rationale for adjunctive corticosteroids in those with severe PCP. Five to 7 days of treatment may be required before a clinical response is observed, defined as a reduction in fever, and improvement in hypoxemia, respiratory symptoms, and radiographic abnormalities. Adverse reactions to treatment are common in patients with AIDS. Many patients can be treated through these with supportive care and adjunctive medications to ameliorate symptoms; however, severe adverse reactions may require substituting alternative treatment regimens. Adverse effects most often associated with TMP-SMX include skin rash (rarely, Stevens-Johnson syndrome or toxic epidermal necrolysis), fever, hepatotoxicity, leukopenia, thrombocytopenia, renal dysfunction, and hyperkalemia. Major side effects of pentamidine include pancreatitis, renal dysfunction, dysglycemia, electrolyte abnormalities, and cardiac dysrhythmias. Toxicities commonly observed with trimetrexate include bone marrow suppression (particularly in those who are not treated with leukovorin), fever, rash, and hepatitis. Dapsone may cause fever, skin rash, and methemoglobinemia with hemolysis (particularly in those with G-6-PD deficiency). Primaquine also causes methemoglobinemia and anemia, and clindamycin toxicities include nausea, rash, hepatitis, Clostridium difficile toxin-associated diarrhea, and rarely toxic megacolon. Atovaquone is associated with nausea, vomiting, diarrhea, rash, fever, and hepatitis. Is secondary prophylaxis or maintenance therapy required after treatment of the acute episode of PCP? All patients with a CD4 count of <200 cells/mm3 should receive primary prophylaxis to prevent PCP. All patients who have experienced an episode of PCP should receive secondary prophylaxis to prevent a recurrence of PCP. Prophylaxis should be continued for life unless immune recovery occurs with ART. Prophylaxis for PCP should be discontinued if the CD4 count increases to levels of >200 cells/mm3 and is sustained for at least 3 months. Prophylaxis should be restarted if the CD4 count declines to <200 cells/mm3 or if PCP recurs regardless of CD4 cell count. Table 9-3 summarizes recommended and alternative regimens for primary and secondary prevention of PCP. It should be noted that TMP-SMX and dapsone plus pyrimethamine are also adequate for prevention of toxoplasmosis in susceptible individuals; dapsone alone, atovaquone alone, and aerosolized pentamidine are not.
Mycobacterium Avium Comples (MAC) TOP What are the symptoms of MAC? Disseminated M. avium complex disease is a multiorgan infection, usually occurring in patients with AIDS who have CD4 counts of <50 cells/mm3 and principally affecting blood and lymphoreticular organs (lymph nodes, spleen, liver, bone marrow). Symptoms are nonspecific, usually consisting of high fevers, night sweats, weight loss, anorexia, and fatigue. Hepatosplenomegaly and less commonly central or peripheral lymphadenopathy are seen. Respiratory tract disease with interstitial or occasionally lobar or cavitary pulmonary infiltrates is a less common manifestation but may be seen in the context of multiorgan infection. Although there are no specific laboratory abnormalities associated with disseminated MAC, isolated elevation of alkaline phosphatase, marked anemia, and leukopenia or thrombocytopenia secondary to bone marrow infiltration may occur. The diagnosis is confirmed by the isolation of the organism from a biopsy or a sample obtained for culture from blood or involved tissue; 80%-85% of those with disseminated multiorgan disease have a positive mycobacterial blood culture. Blood cultures may require 7-10 days of incubation before growth of M. avium is detectable. What is the recommended approach to treatment for disseminated MAC? Disseminated MAC should be treated with a combination of clarithromycin 500 mg twice a day or azithromycin 500-600 mg/day plus ethambutol 15 mg/kg/day. The addition of rifabutin, 300-450 mg/day, as a third drug decreases the occurrence of relapse due to drug-resistant mycobacteria and is associated with improved survival in profoundly immunosuppressed individuals. This approach should be considered for those with very low CD4 cell counts who are not receiving or responding to ART. Treatment should be continued for life unless immune recovery occurs following ART that results in an increase in CD4 cells to levels >100/mm3 sustained for at least 6 months. In this setting, antimycobacterial therapy for those who have completed at least 12 months of effective treatment and have no signs or symptoms of disease may be discontinued. Therapy should be re-started if the CD4 cell count again declines to <100/mm3. Primary prophylaxis to prevent disseminated M. avium complex disease is recommended for all HIV-infected adults and adolescents who have a CD4 count of <50 cells/mm3. The preferred regimens are azithromycin 1200 mg once a week or clarithromycin 500 mg twice a day. Azithromycin is preferred when patients are receiving other drugs metabolized by the cytochrome P450 3A4 isoenzyme system, to avoid drug-drug interactions with clarithromycin. Rifabutin 300 mg/day is a second line alternative for prophylaxis, but is less effective than azithromycin or clarithromycin, and also must be monitored and dose-adjusted when used with other drugs metabolized by the cytochrome 3A4 isoenzyme. Cytomegalovirus Infection (CMV) TOP What are the major clinical syndromes associated with CMV infection in patients with HIV infection and how are they diagnosed? Cytomegalovirus retinitis is the most common CMV-associated clinical syndrome observed in patients with AIDS. Patients with CMV retinitis involving peripheral portions of the retina may have no symptoms, or only minor visual complaints such as floaters or peripheral visual field defects. Central lesions may result in central scotomata and loss of central vision. The diagnosis is based on ophthalmologic examination demonstrating creamy white retinal exudates with hemorrhage; lesions obscure visualization of underlying vessels and other retinal structures. Retinitis is bilateral in up to 20% of patients. Disease progresses rapidly to blindness unless treated. The second-most common clinical syndrome associated with CMV disease is gastrointestinal disease, either CMV colitis or CMV esophagitis. CMV colitis presents with fever, diarrhea, weight loss, and colon ulceration often with bleeding. Appropriate diagnosis usually requires colonoscopy and biopsy of ulcerations; tissue histopathology confirms the presence of characteristic intranuclear inclusions in mucosal cells or CMV antigens on immunohistochemical staining. CMV esophagitis presents with fever, odynophagia, and retrosternal pain. Exudative ulcers are demonstrated by esophageal endoscopic exam. Biopsy and histopathologic examination are necessary to confirm the diagnosis. Other less common CMV clinical syndromes in patients with AIDS are CMV encephalitis/encephalopathy, interstitial pneumonitis, hepatitis, and pancreatitis. What is the recommended treatment for CMV retinitis? CMV retinitis should be managed in cooperation with an experienced ophthalmologist. For patients with CMV retinitis that is immediately sight-threatening (central lesions approaching or involving the optic nerve or the fovea) systemic therapy with either IV ganciclovir or oral valganciclovir is the preferred initial treatment; many experts would also recommend surgical treatment with a ganciclovir intraocular implant (see Table 9-4). IV ganciclovir or oral valganciclovir is administered in an acute "induction" dose for 14-21 days, followed by a once-a-day chronic maintenance therapy dose. This approach is superior to other alternatives for preventing or delaying disease progression and provides systemic treatment to prevent other organ system involvement. If effective ART is not administered or patients remain immunosuppressed, the ocular implant should be replaced every 7-8 months. For patients with peripheral CMV retinitis that is not immediately sight-threatening, oral valganciclovir is the preferred treatment because of its ease of administration compared with other parenteral alternatives. Chronic maintenance treatment for CMV retinitis should be continued for life unless ART results in a sustained increase in CD4 counts to levels of >100 cells/mm3 for at least 6 months. If the CD4 count again declines to <100 cells/mm3, maintenance therapy should be reinitiated. Immune recovery vitritis is a recognized complication of acute rises in CD4 cell counts and decreases in plasma HIV RNA levels following initiation of ART. It may be associated with an acute decrease in visual acuity due to the inflammatory cells clouding the vitreous fluid. Treatment with local corticosteroids while continuing anti-CMV therapy is associated with rapid improvement in most patients.
What is the recommended treatment for CMV gastrointestinal disease? Treatment with
either oral valganciclovir or IV ganciclovir or IV foscarnet is
the recommended approach to treatment of CMV esophagitis or CMV
colitis. In general, clinical symptoms and signs resolve within
2-3 weeks, and the suggested duration of treatment is 21-28 days,
depending on the severity of the clinical presentation. Chronic
maintenance treatment is not recommended unless recurrent episodes
occur. Candida Esophagitis TOP What are the presenting signs and symptoms of Candida esophagitis and how is it diagnosed? The most common manifestations of Candida esophagitis are fever, anorexia, odynophagia, and retrosternal pain. Occasionally, nausea and rarely UGI bleeding from ulceration may occur. Esophagoscopy reveals adherent whitish plaques with shallow ulcers surrounded by erythematous borders. Invasive esophageal candidiasis can occur in the absence of oral candidiasis and must be distinguished from esophagitis due to CMV, herpes simplex virus, and aphthous esophagitis, all of which may be seen in patients with HIV infection. Isolation of Candida species in culture from a specimen obtained from the mucosal surface or the base of an ulcer provides supportive information but is not diagnostic, as colonization of mucosal surfaces with Candida is common in immunosuppressed individuals, particularly those who are receiving antibacterial therapies such as TMP-SMX for prophylaxis of PCP. The diagnosis can be confirmed by a biopsy of the ulcer and demonstration of yeast forms in tissue. The finding of oral candidiasis associated with esophageal symptoms has a positive predictive value of close to 100% for Candida esophagitis. In patients with oral candidiasis and esophageal symptoms empiric therapy for Candida esophagitis should be given. If there is no clinical response after 5-7 days, upper endoscopy should be performed. How do you treat Candida esophagitis? Candida esophagitis requires systemic therapy. A number of options are available; however, the preferred treatment is fluconazole 100 mg/d for 14 days. Ketoconazole and itraconazole are effective alternatives, but drug-drug interactions with other anti-HIV therapies are common, and absorption is diminished in those with primary or drug-induced hypo- or achlorhydria. For patients unable to tolerate oral therapy, parenteral amphotericin B is effective. Chronic maintenance therapy or secondary prophylaxis is not recommended, but may be considered for patients who have frequent recurrent episodes. Chronic suppressive therapy with fluconazole has been associated with an increased risk of fluconazole (and other azole) resistant disease. Central Nervous System Diseases TOP How do you distinguish cryptococcal meningitis from toxoplasmic encephalitis and CNS lymphoma in HIV-infected patients? Patients with AIDS and a CD4 count of <200 cells/mm3 may be at risk for developing CNS OIs such as cryptococcal meningitis or toxoplasmosis, as well as CNS lymphoma; most infections occur in those with CD4 counts of <100 cells/mm3. Toxoplasmic encephalitis is rare in patients who are seronegative for Toxoplasma gondii (negative IgG antibody). CNS infection due to T. gondii and Cryptococcus spp. as well as CNS lymphoma may be relatively insidious in onset and associated with symptoms of fever, headache, and mental status changes. Focal neurological deficits and seizures are more likely to occur with toxoplasmic encephalitis and CNS lymphoma, although they sometimes occur in those with cryptococcal meningitis as well. The best diagnostic tests for a differential diagnosis of CNS disease are radiographic imaging of the CNS (with either a contrast-enhanced computerized tomographic [CT] scan or magnetic resonance imaging [MRI] scan) and lumbar puncture with cerebrospinal fluid (CSF) examination. Multiple localized enhancing lesions are common with toxoplasmic encephalitis; MRI is more sensitive than a CT scan for detecting these. CNS lymphoma is more likely to be associated with single and less commonly multiple space-occupying lesions, while cryptococcal meningitis is only rarely associated with space-occupying lesions. The CSF may demonstrate a normal to mild lymphocytic pleocytosis, normal to slightly elevated protein, and normal to mildly decreased glucose for each, but a CSF cryptococcal antigen will be positive in 99%-100% of individuals with cryptococcal meningitis. Confirmation of the diagnosis of toxoplasmic encephalitis generally requires either demonstration of the organism in CSF by polymerase chain reaction (PCR) or culture (may not be routinely available) or a brain biopsy and demonstration of the organism in tissue. In practice, a trial of therapy is usually the first diagnostic tool for patients who have suggestive neuroimaging and positive Toxoplasma serology. Brain biopsy is reserved for patients who do not respond to empiric therapy. A brain biopsy is necessary to confirm the diagnosis of CNS lymphoma. How do you treat cryptococcal meningitis? Amphotericin B (0.7 mg/kg/d or the dose equivalent of liposomal or lipid complex amphotericin B), combined with flucytosine 100 mg/kg/day in divided doses, for the first 2 weeks, is the recommended initial treatment for cryptococcal meningitis. If a treatment response is evident this regimen is followed by fluconazole 400 mg/day for an additional 8 weeks or until CSF cultures are negative. The CSF opening pressure should always be measured when a lumbar puncture is performed, and if elevated above 200 mg H2O, measures to reduce the pressure should be implemented. These may include serial lumbar puncture with CSF drainage sufficient to reduce the pressure by 50%, or in the case of severe increased intracranial pressure, the use of a lumbar drain or ventriculoperitoneal shunt. Secondary prophylaxis or chronic maintenance therapy with fluconazole, 200 mg/day, should be continued for life unless ART results in immune recovery. In this instance, maintenance therapy may be discontinued for those who have successfully completed a course of therapy for cryptococcal meningitis, have no residual signs or symptoms, and have a sustained increase in CD4 count to levels of >100-200 cells/mm3 for at least 6 months. Maintenance therapy should be re-initiated if the CD4 count again decreases to <100-200 cells/mm3. Mycobacterium Tuberculosis TOP Does the presentation of tuberculosis differ in HIV-infected and non-HIV-infected individuals? Patients with
HIV infection have an increased risk of infection with Mycobacterium
tuberculosis following exposure to a person with active disease,
and an increased risk of developing active disease following an
exposure. The risk of developing active disease is 30%-40% in the
first year following a new exposure, and for HIV-infected patients
with a positive tuberculin skin test related to prior TB exposure,
the risk of developing active disease may be as high as 7%-8% per
year. How do you diagnose TB in persons with HIV? The diagnosis is the same as for non-HIV-infected persons, relying on a combination of compatible clinical signs and symptoms, radiographic findings, a positive tuberculin skin test, and a sputum sample demonstrating acid-fast bacilli (AFB). The diagnosis is confirmed by culture of the organism from sputum, blood, or other tissue. However, patients with advanced immunosuppression may have less extensive consolidation or lung cavitation, and sputum AFB smears may be positive in a lower proportion, because of the lower mycobacterial burden in the lung. Also, tuberculin skin tests are less likely to be positive because of impaired cell-mediated immune function. In immunocompromised patients, more aggressive means for making a diagnosis may be necessary, such as bronchoscopy with bronchoalveolar lavage or transbronchial biopsy to obtain samples for AFB smear and culture. Biopsy or culture of specimens obtained from extrapulmonary sites such as bone marrow and lymph nodes may be useful. All initial isolates of M. tuberculosis should be tested for susceptibility to antimycobacterial drugs, and susceptibility testing should guide the choice of initial treatment. How do you treat tuberculosis in patients with HIV infection? The recommendations for treatment of tuberculosis in patients with HIV infection depend in part on whether or not patients are on ART, and if they are, the specific antiretroviral regimens employed. All patients should receive directly observed therapy. See Adult OI Tables 2 - 7 for the recommendations for treatment of latent tuberculosis and drug-susceptible tuberculosis disease. Treatment with 4 drugs (isoniazid, rifampin or rifabutin, ethambutol, and pyrazinamide) is recommended either for the first 2 months of therapy or until results of susceptibility testing are available. If patients have drug-susceptible disease, 2 drugs, isoniazid and rifampin or rifabutin, should then be continued for at least 18 additional weeks. The continuation phase of therapy should be extended to 28 weeks (total duration of 9 months) for HIV-infected patients with advanced immunosuppression (CD4 count of <100 cells/mm3) or who have an inadequate initial response or cavitary disease. Rifabutin should replace rifampin and doses should be appropriately adjusted for those who are receiving ART with agents likely to interact with rifamycins. The timing of initiation of ART and the most appropriate ART regimen for use in conjunction with antimycobacterial therapy remain controversial. Concurrent ART and tuberculosis treatment should be managed by an expert. Malignancies TOP How does Kaposi sarcoma (KS) present? KS is a poorly understood multicentric tumor of endothelial cells. The cause is human herpesvirus 8 (HHV-8), an infection that is more frequent in men who have sex with men. The mechanism of transmission is unclear, but the best evidence suggests transmission via saliva and semen. Many carry the virus with no consequences; it causes disease primarily in the presence of immunosuppression. The presentation is highly characteristic, with multiple purple to brown-black macules, or nodules that are usually asymptomatic and occur most frequently on the legs, face, oral cavity, or genitalia. The major complications include lymphedema (especially of the legs, face, and genitalia) and visceral involvement. Visceral involvement is common in the lung and GI tract, but is usually asymptomatic. How do you diagnose KS? The skin lesions are usually sufficiently characteristic to make this diagnosis clinically based on appearance, but a biopsy should be done when there is a need for confirmation. The differential diagnosis includes bacillary angiomatosis (which can be proven by biopsy with silver stain), hematoma, nevus, hemangioma, B-cell lymphoma, and pyogenic granuloma. When there is visceral involvement, the usual method used to establish the diagnosis is endoscopic examination to show typical mucosal surface lesions. With lung involvement, the x-ray is variable and may show nodules, infiltrates, effusions, and/or mediastinal/hilar nodes. Lung biopsy is often negative, but the bronchoscopic exam often shows a characteristic cherry-red bronchial nodule. With GI tract involvement, the usual screening test is stool for occult blood, and endoscopy is the usual method to make the diagnosis by showing a hemorrhagic nodule; the biopsy is often negative because of the submucosal location of the tumor. How do you treat KS? Many patients require no therapy except possibly makeup to cover the characteristic lesions. Topical treatment for skin involvement includes vinblastine, Panretin gel, liquid nitrogen, radiation, cryosurgery, or laser. Systemic chemotherapy is preferred when there is an extensive burden, defined as >25 skin lesions, symptomatic visceral involvement, extensive edema, systemic (B) symptoms, or failure to respond to local treatment. The favored forms of chemotherapy are liposomal anthracyclines (Doxil or DaunoXome) or, less frequently, paclitaxel (Taxol). Immune reconstitution with ART is associated with a substantial reduction in the frequency of KS and a therapeutic improvement in those who already have these lesions. It should be noted that there is no cure for KS, and the goal of local therapy or systemic chemotherapy is to reduce tumor burden and relieve symptoms. What lymphomas are associated with HIV infection? The subtypes of lymphomas associated with HIV infection include B-cell large cell lymphoma, primary body effusion lymphomas, B-cell CNS lymphoma, Burkitt lymphoma, plasmablastic lymphoma, and Hodgkin disease. The most common is non-Hodgkin lymphoma. What are the symptoms of non-Hodgkin lymphoma (NHL)? Compared with the general population, patients with this complication have high rates of stage IV disease with systemic (B) symptoms and sparse node involvement. Common symptoms are fever of unknown origin, liver dysfunction, marrow suppression, lung disease (effusions, multinodular infiltrates, mass lesions, diffuse infiltrates, and/or hilar adenopathy), GI involvement (any level with pain and weight loss), and CNS with mass lesions. How do you diagnose lymphomas? Fine needle aspirates of enlarged nodes are helpful if positive, but false negatives are common. Biopsy is usually necessary. How are lymphomas treated and what should be expected? The usual treatment is chemotherapy with cyclophosphamide, doxorubicin, adriamycin, vincristine, and prednisone (CHOP); methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone + G-CSF (M-BACOD); or etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH). Response rates are 50%-60%, but the long-term prognosis is poor unless there is immune reconstitution with ART. How do you diagnose and treat primary CNS lymphomas? The typical presentation is confusion, headache, memory loss, focal neurologic changes, usually without fever and usually with a CD4 cell count of <50/mm3. The MRI shows single or multiple lesions that are isodense or hypodense. The differential diagnosis is primarily toxoplasmosis. Factors suggesting CNS lymphoma are the characteristic MRI findings, negative T. gondii serology, failure to respond to empiric treatment of toxoplasmosis within 1-2 weeks, thallium single-photon emission computed tomography (thallium SPECT) scan results, and CSF positive by polymerase chain reaction (PCR) for Epstein Barr virus (EBV) DNA. The definitive diagnosis can be made by stereotactic brain biopsy. Treatment is radiation and corticosteroids, often combined with chemotherapy. The prognosis is poor without immune reconstitution.
Suggested Resources TOP Leav BA, Mackay
M, Ward HD. "Cryptosporidium species: New insights Official statement of ATS, CDC and IDSA: Treatment of tuberculosis. Am J Crit Care Med. 2003;167:603-662. Accessed 1/04. USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected with HIV. Washington DC: U.S. Department of Health and Human Services. Nov 28, 2001. Accessed 1/04. |
||||||||||||||||||||||||||||||||||||||||||||||||
![]()
Top
| Home | HRSA
| HHS |
Disclaimer | Accessibility
| Privacy | Download
Adobe Reader| | Freedom of Information
Act