DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
Statement by
Richard J. Hodes, M.D.
Director, National Institute on Aging
on
Fiscal Year 2001 President's Budget Request for the National Institute on Aging
Director's Statement
Alzheimer’s Disease Research
Biology of Aging
Reducing Disease and Disability
Reducing Health Disparities
Chart 1: The Anatomy of Memory
Chart 2: Production of Amyloid Plaques
Chart 3: Longevity Genes Across Species
Chart 4: Becoming Physically Fit Reduces Mortality in Elderly Persons
Director's Statement
Mr. Chairman and Members of the Committee:
I am pleased to present the President’s non-AIDS budget request for the National Institute on Aging (NIA) for FY 2001, a sum of $721,651,000, which reflects an increase of $37,933,000 over the comparable Fiscal Year 2000 appropriation. Including the estimated allocation for AIDS, total support requested for the National Institute on Aging is $725,949,000, an increase of $38,088,000 over the Fiscal Year 2000 appropriation. Funds for NIA efforts in AIDS research are included within the Office of AIDS Research budget request.
The NIH budget request includes the performance information required by the Government Performance and Results Act (GPRA) of 1993. Prominent in the performance data is NIH’s first performance report which compares our FY 1999 results to the goals in our FY 1999 performance plan. As our performance measures mature and performance trends emerge, the GPRA data will serve as indicators to support the identification of strategies and objectives to continuously improve programs across the NIH and the Department.
Since the NIA’s inception in 1974, tremendous strides have been made in uncovering the mysteries of the aging process, reducing disease and disability, and improving the quality of life for older Americans. The pace of scientific discovery has been impressive, and the Institute anticipates building upon these advances in the future. I am pleased to report the NIA’s recent progress, in reducing disability and extending healthy active years of life for all Americans.
Alzheimer’s disease (AD), the most common cause of dementia, is the result of abnormal changes in the brain that lead to a devastating decline in intellectual abilities and changes in behavior and personality. Tragically, as many as four million Americans now suffer from Alzheimer’s disease—a number that threatens to increase dramatically as the population of people most at risk for dementia, those aged 85 and older, reaches almost 20 million by the middle of the 21st century. NIA, as the lead Federal agency responsible for Alzheimer’s disease research, recognizes the urgency of this looming public health threat and is supporting basic, clinical, and behavioral research to improve AD diagnosis, treatment, and patient care, and to delay, and eventually prevent, the onset of this devastating disease. Advances in our understanding of AD over the last 20 years have been substantial, enabling the NIA to launch the Alzheimer’s Disease Prevention Initiative. In collaboration with other Federal agencies and the private sector, the AD Prevention Initiative is invigorating discovery of new treatments, risk and preventative factors, methods of early detection and diagnosis; and strategies for improving patient care and alleviating caregiver burdens. The initiative is also accelerating movement of promising new treatments and prevention strategies into clinical trials, and is improving understanding of normal brain function.
In 1999, the NIA launched the first large-scale AD prevention clinical trial supported by the NIH, the Memory Impairment Study (MIS), being conducted at more than 65 medical research institutions in North America. In this trial, vitamin E, and donepezil (Aricept) will be evaluated over a three-year period for their effectiveness in slowing or stopping the conversion from mild cognitive impairment (MCI), a condition characterized by a memory deficit without dementia, to AD. Other ongoing or upcoming AD prevention trials will examine the effectiveness of ibuprofen (an anti-inflammatory drug) in reducing the development of AD; the effect of estrogen replacement therapy in preventing AD in women with a family history of the disease; and whether treatment with a variety of agents, such as aspirin, vitamin E, antioxidants, or combined folate/B6/B12 supplementation can prevent AD. The effects of these agents on normal age-related decline will also be evaluated. Information about ongoing clinical trials is available to the public through the NIA-supported Alzheimer’s Disease Education and Referral Center web site (www.alzheimers.org) and toll-free number (1-800-438-4380).
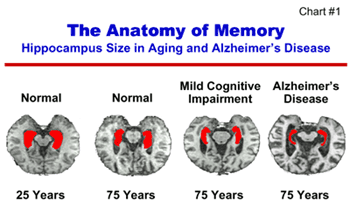
The ability to assess the effectiveness of early treatments or interventions, such as those being tested in the AD Prevention Initiative, will be enhanced by our ability to visualize brain function using new imaging techniques. In a recent study, investigators used magnetic resonance imaging (MRI) to determine volume measurements of the hippocampus, the region of the brain responsible for memory function, in individuals diagnosed with mild cognitive impairment (Chart #1). Based on three years of observations, researchers found that in older people with MCI, the smaller the hippocampus at the beginning of the study, the greater the risk of developing AD later. This imaging study illustrates how abnormal cerebral function or anatomy can be detected before clinical diagnosis and how diagnostic advances can help ensure the effective application of emerging early interventions. Advances in imaging techniques also have important diagnostic implications for other neurodegenerative diseases, such as Parkinson’s disease.
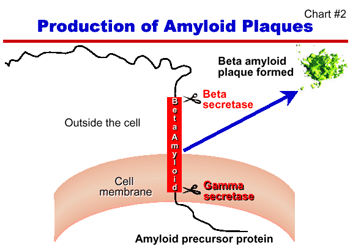
Developing effective treatments for AD based on advances in basic research is a major focus of NIA-supported studies. The ability of researchers to conceptualize effective treatments was enhanced by the discovery of two enzymes, beta and gamma secretase, that are involved in the clipping of a normal cell surface protein to produce the amyloid peptide that forms the senile plaques found in the brains of AD patients (Chart #2). Identifying and understanding how these two enzymes work will accelerate the development of interventions to block their action and stop the development of AD plaques. NIA will also support research to evaluate the potential of an immunization approach recently developed by researchers in the private sector, which, in mice, prevented the formation of amyloid plaques associated with AD.
A transgenic mouse strain that expresses a human tau gene and develops AD-like tau tangles has been developed. This model will help scientists understand how tau produces AD in the brain, and together with other AD models, will move researchers closer to developing effective preventive or treatment interventions. In another study, researchers demonstrated that shrinkage and dysfunction of certain brain cells that occur with age might be reversible. Researchers inserted into skin cells a gene that makes human nerve growth factor (NGF) and then injected the modified cells into the brains of experimental animals. After three months, the older animals injected with NGF-expressing cells had brains that resembled those of younger animals. Such gene transfer approaches to recovering cellular function could eventually have important implications for the treatment of AD and other chronic age-related neurodegenerative disorders in humans.
Research on the biology of aging has led to a revolution in understanding the cellular and molecular changes that occur with aging and the abnormal changes that are risk factors for or accompany age-related diseases. Further, research is revealing genetic and other biologic factors associated with extended longevity in animal models, contributing to the development of interventions to reduce or delay age-related degenerative processes in humans. Presently, caloric restriction is the only intervention known to slow the intrinsic rate of aging in mammals. Rodents and other laboratory animals that are given a diet that includes necessary nutrients, but 30 to 40 percent fewer calories than in usual diets, live far beyond their normal life spans and have reduced rates and later onset of diseases. A recent study analyzed the gene expression profiles of cells from young and from old mice on usual or calorically restricted diets. Of the 6,347 genes surveyed by new micro-array techniques, fewer than one percent displayed a greater than twofold decrease in expression. Thus, the aging process may be associated with changes in expression of a limited subset of genes, rather than involving widespread changes in most genes. It was further observed that caloric restriction completely or partially suppressed age-associated alterations in expression of a large proportion of genes. This type of molecular assessment of mammalian aging will provide new tools to evaluate experimental interventions for age-related conditions.
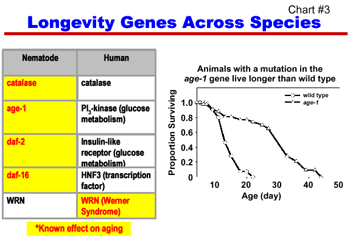
Over the last ten years, numerous genes have been implicated in normal aging processes, in age-related pathologies and diseases, and in determination of longevity in several species including humans (Chart #3). Understanding the molecular genetics of aging and longevity is a rapidly advancing field; recent research advances have greatly expanded our knowledge of genes and biological pathways which play significant roles in determining longevity and health span. Recent discoveries in non-mammalian species including S. cerevisiae (yeast), C. elegans, (roundworms), and D. melanogaster, (fruit flies), have identified striking effects of mutations, either singly or in specific combinations, on lifespan, increasing life spans 2 to 3 times longer than those of (wild type) normally aged animals. These findings suggest signaling and metabolic pathways that may be critical in determining longevity. In addition, cross-species comparisons have identified homologous (similar) genes in animals and in humans that have similar or related functions. For example, mutations that double the life span of C. elegans occur in genes that are homologous to genes associated with insulin and glucose (sugar) metabolism in humans, and may therefore be relevant to weight control and diabetes.
Reducing Disease and Disability
Studies have shown that disability rates for people age 65 and older have been falling at an accelerating pace since 1982 and that the benefits of this trend extend to both men and women, and to minority groups. Initial field reports from the 1999 wave of the National Long-Term Care Survey indicate that disability rates have continued to fall. These data parallel heartening news from investigators who found that many centenarians remain functionally independent for the vast majority of their lives. More research is needed to understand the genetic and environmental factors responsible for centenarians’ prolonged good health and extreme longevity. Similarly, more research is necessary to understand the causes and economic consequences of the decline in disability rates and to further accelerate these improvements.
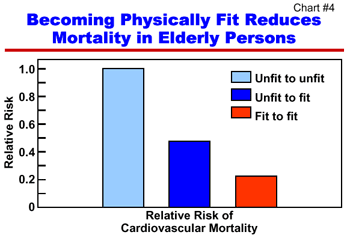
Increasingly, researchers are understanding the benefits of exercise, especially for older people, as a key to preventing or delaying the onset of disease and disability. Specifically, studies have revealed that moderate physical activity can: reduce the risk of falls; benefit people suffering from a variety of ailments, including osteoarthritis and depression; and may enhance learning, memory and the generation of brain cells. There is also scientific evidence that exercise may be a factor related to increased life expectancy and the number of years people live free of disability. In a recent study, it was shown that becoming fit, even in later years (as measured by performance on an exercise treadmill test), is associated with lower mortality rates (Chart #4). The study, which included 9,000 men aged 20 to 82, compared death rates in physically unfit men who remained unfit over five years with physically unfit men who became fit during the same period. The study found that unfit men aged 60 and over who became fit had death rates 50 percent lower than those who remained unfit. In another clinical trial involving chronically ill older adults, aged 70 and older, researchers reported that one year of increased physical activity, combined with chronic-illness self-management resulted in fewer reported hospitalizations and total hospital days. These studies show that exercise can benefit older people and that it is never too late to start. The challenge remains to motivate more people to engage in regular physical activity—particularly older women and minorities who, according to national surveys, are consistently less active. Last year, the National Institute on Aging published a free manual, Exercise: A Guide from the National Institute on Aging, the cornerstone of the Institute's ongoing campaign to encourage older people to exercise. The Guide is based on scientific evidence and is intended to help people design their own exercise program. To date, the Institute has distributed over 230,000 copies.
Reducing Health Disparities
Health disparities are associated with a broad, complex, and interrelated array of factors, including race, ethnicity, gender, socioeconomic status, age, education, occupation, and as yet unknown lifetime and lifestyle differences. Prevalence rates of specific diseases are also associated with race, ethnicity and socioeconomic status. One recent multi-ethnic epidemiologic study indicated that prevalence rates for Alzheimer's disease may be higher for African-Americans and Hispanics. In an examination of genetic risk factors for AD, although APOE4 is associated with higher risk of AD among Caucasians, African Americans and Hispanic elders with the APOE4 allele were not at higher risk than those with other APOE alleles. Understanding the genetic and environmental risk factors contributing to this difference will be crucial to developing effective interventions to reducing the disparity.
NIA has made reducing health disparities a major priority of its five-year strategic plan. Last year, with support from the NIH Office of Research on Minority Health, the NIA intramural program designed a mobile medical research vehicle to extend the opportunity to participate in the Baltimore Longitudinal Study of Aging and in other clinical research projects to minority and socioeconomically diverse subjects. The NIA, working with the National Advisory Council on Aging, also undertook a comprehensive review of its minority aging research activities and training initiatives. The NIA is developing an implementation plan to integrate the resulting recommendations into its programs, such as the Resource Centers for Minority Aging Research, which provide mentoring and training opportunities to individuals interested in studying the health of minority elders, and the Alzheimer’s Disease Centers satellite clinics, which recruit minorities for research protocols conducted by the NIA’s 28 AD centers.
Investigators supported by the NIA continue to report exciting scientific advances that may help eliminate health disparities among groups of elders. Investigators recently published a study, which suggests that there is a difference between African-American and Caucasian women in their experience of perimenopausal symptoms. African-American women were significantly more likely than white women (58% vs. 29%) to experience hot flashes, but fewer than 7% had discussed menopausal management with their physicians. Given our developing understanding of hormone replacement therapy in controlling menopausal symptoms as well as in reducing risk of problems in later life, such as osteoporosis, improved, culturally appropriate patient education programs should be encouraged.
A decade ago, few interventions were available to address the major health concerns of older people. Entering the 21st century, the outlook has improved considerably. More interventions exist and research into treating, as well as preventing, the onset of age-related diseases is escalating. With the knowledge this research will derive, aging will be a healthier, more vigorous stage of life. I am happy to answer your questions.
