
Volume 131, Issue 3, 2 November 2007, Pages 429-432
| This article originally appeared in Cell and is presented with permission from that publication. |

Volume
131, Issue 3, 2 November 2007, Pages 429-432
Commentary
25 Years of HIV/AIDS Science: Reaching the Poor with Research Advances
Anthony S. Fauci1,
 ,
, 
Last year marked the 25th anniversary of the recognition of what we now call AIDS. The AIDS pandemic has claimed more than 25 million lives, the majority of them in the developing world, and has exacerbated poverty and slowed human development. Although much has been accomplished in HIV/AIDS research, much remains to be done, especially regarding delivery of HIV/AIDS therapies and care and prevention interventions to the poorest countries that need them most.
By the end of 2006—25 years after the first reported AIDS cases in the United States—the HIV/AIDS pandemic had claimed more than 25 million lives globally (UNAIDS/WHO, 2006). What began as a handful of recognized cases among homosexual men in the early 1980s has become a global pandemic of such proportions that it clearly ranks as one of the most destructive microbial scourges in history ([UNAIDS, 2006] and [Fauci, 2006]).
This Commentary is part of the Council of Science Editors' Global Theme Issue on Poverty and Human Development comprising 1000 articles on this theme published simultaneously by 235 journals worldwide.
Of the 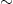 40
million people living with HIV, the vast majority live in resource-poor
settings, notably countries of sub-Saharan Africa where food insecurity and
endemic infections such as tuberculosis, malaria, and a range of parasitic
diseases are also common (UNAIDS,
2006). Of the 62 countries most affected by HIV (nations with adult HIV
seroprevalence rates >1% or with large numbers of persons living with HIV),
40 are in sub-Saharan Africa, 5 in Asia, 12 in the Americas and the Caribbean, 4
in Europe, and 1 in Oceania (United
Nations Population Division, 2006). According to the United Nations
Population Division, life expectancy in southern Africa as a whole has fallen
from 61 to 49 years over the last 20 years. In the countries hardest hit by
HIV/AIDS, the situation is even more severe: in Zimbabwe, for example, estimated
life expectancy at birth is only 34 years for women and 37 years for men.
40
million people living with HIV, the vast majority live in resource-poor
settings, notably countries of sub-Saharan Africa where food insecurity and
endemic infections such as tuberculosis, malaria, and a range of parasitic
diseases are also common (UNAIDS,
2006). Of the 62 countries most affected by HIV (nations with adult HIV
seroprevalence rates >1% or with large numbers of persons living with HIV),
40 are in sub-Saharan Africa, 5 in Asia, 12 in the Americas and the Caribbean, 4
in Europe, and 1 in Oceania (United
Nations Population Division, 2006). According to the United Nations
Population Division, life expectancy in southern Africa as a whole has fallen
from 61 to 49 years over the last 20 years. In the countries hardest hit by
HIV/AIDS, the situation is even more severe: in Zimbabwe, for example, estimated
life expectancy at birth is only 34 years for women and 37 years for men.
In addition to the enormous human tragedy associated with HIV/AIDS, the economic costs are staggering, posing serious impediments to the growth and stability of many developing countries (Bertozzi et al., 2006). HIV/AIDS has disproportionately affected young and middle-aged adults who are the mainstays of economies and the breadwinners for families. In many developing countries, previously realized gains in economic and social development have been reversed as HIV/AIDS has reduced the labor supply and productivity and depleted limited pools of skilled workers, teachers, and managers. In communities with large numbers of HIV-infected individuals, poverty and other development challenges have worsened. An exacerbation of poverty in turn reduces job opportunities and makes populations more vulnerable to the spread of HIV.
As the humanitarian and economic burden of HIV/AIDS has grown, so too has the biomedical research response to the pandemic, providing important opportunities to mitigate the impact of HIV/AIDS on health and economic development. Funding for HIV/AIDS-related research is unprecedented in magnitude, larger than for any other single disease in history (Folkers and Fauci, 2001). At the US National Institutes of Health alone, >$30 billion has been spent on HIV/AIDS research since the beginning of the pandemic (Fauci, 2006). The returns on these investments have been substantial (reviewed in Fauci, 2003). Within 3 years of the recognition of the first AIDS cases in 1981, the etiologic agent of the syndrome was discovered and causality proven. In 1985, a sensitive and specific diagnostic assay for HIV antibodies was developed. This test and its descendents have been used in epidemiological studies to illuminate the scope of the epidemic, as well as to safeguard blood supplies. Molecular studies of HIV have delineated the genetic and structural organization of the virus and the mechanisms that regulate its replication. This information and related research on HIV pathogenesis has facilitated the rapid development of antiretroviral drugs (ARVs) that limit HIV replication and immune system damage, as well as tools to monitor levels of virus in the blood and immune status. More than 25 antiretroviral drugs are now available to treat HIV infection, and these medications have had an enormous impact in reducing morbidity and mortality wherever they have been used (WHO/UNAIDS/UNICEF, 2007). New ARVs such as integrase, maturation, and entry inhibitors, as well as next-generation reverse transcriptase inhibitors and protease inhibitors, have been licensed or are in late-stage clinical development. These drugs hold great promise, especially for patients who have failed their current regimens due to drug resistance or other factors (Siegel and Gulick, 2007).
Encouragingly, growing numbers of HIV-infected individuals in need of ARVs in low- and middle-income countries are receiving them. A growing body of evidence has shown that the immunological and virological responses to ARVs in both adults and children in poor countries can be as good as those in industrialized settings. ARV prices have fallen dramatically: the average annual cost in low- and middle-income countries of a common first-line ARV regimen consisting of 3 medications was <$150 in 2006. Programs such as the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), the Global Fund to Fight AIDS, Tuberculosis, and Malaria, nongovernmental organizations such as Medecins Sans Frontieres, and philanthropies like the Bill and Melinda Gates Foundation and the Clinton Foundation helped deliver ARVs to >2 million individuals by the end of 2006, up from 400,000 in December, 2003 (see Figure 1) (WHO/UNAIDS/UNICEF, 2007). This progress demonstrates what can be accomplished even in the developing world with increased funding, a strong global commitment, collective action, and political will.
Figure 1. ARV Therapy for AIDS Patients
Estimated number of HIV-infected people receiving antiretroviral therapy in low- and middle-income countries as of December 2006. (Data from WHO/UNAIDS/UNICEF, 2007.)
Significant progress also has been made in HIV prevention. The risk factors associated with HIV transmission are well defined, and proven methods to prevent sexual HIV transmission (e.g., behavior change programs, condom promotion), blood-borne HIV transmission (e.g., needle/syringe exchanges), and mother-to-child transmission (e.g., ARVs) have been deployed throughout the world (Global HIV Prevention Working Group, 2007). No single intervention is a magic bullet but when deployed in combination can have an important impact, as evidenced by well-documented success in Brazil, Thailand, Uganda, Senegal, and other countries, both rich and poor.
Promising research on new prevention interventions holds hope for adding new tools to the HIV prevention armamentarium, such as adult male circumcision, topical microbicides, pre-exposure chemoprophylaxis with well-tolerated ARVs such as tenofovir, and an HIV vaccine (Bertozzi et al., 2006). A preventive HIV vaccine remains the greatest hope for reversing the relentless spread of HIV, and clinical trials of diverse approaches are ongoing throughout the world (Johnston and Fauci, 2007). Even an “imperfect” HIV vaccine would have enormous benefits. In this regard, progress has been made in developing so-called T cell-based HIV vaccines, which evoke cell-mediated immunity that may not protect against infection but could nonetheless help lower the initial burst of viremia following infection as well as the viral set point and slow disease progression and reduce transmissibility. Meanwhile, structural studies of the HIV envelope may lead to the development of immunogens that elicit broadly neutralizing antibodies that could form the basis of a “sterilizing” vaccine that blocks infection altogether (Johnston and Fauci, 2007).
While extraordinary scientific, medical, and public health accomplishments have been made in the battle against HIV/AIDS, much remains to be done, especially with regard to delivering the fruits of the research endeavor to resource-poor countries where the pandemic continues to destroy lives, communities, and societies in staggering numbers (Global HIV Prevention Working Group, 2007). Evidence-based HIV interventions are needed at high levels of coverage, uptake, intensity, and duration if they are to have a significant public health impact. Priorities include the effective scale-up of HIV/AIDS therapy, care, and prevention, training of health care workers and ancillary personnel, support of orphans and vulnerable children, and the strengthening of health care systems and infrastructure in HIV-burdened regions (UNAIDS, 2006). Each of these will require an increased and sustained infusion of resources from rich countries and other donors. It is encouraging that annual spending on HIV/AIDS in poor- and middle-income countries increased six-fold between 2001 and 2007 to approximately $10 billion, but this sum represents less than half the amount that UNAIDS estimates will be needed in 2008 for the expanded response needed to slow the pandemic.
Despite the progress with ARV access noted above, huge gaps remain in the
provision of these life-saving drugs. Only 28% of people (and just 15% of
children) in need of ARVs in low- and middle-income countries are receiving
them, and problems of access persist in developed countries as well (see Figure
1). A shortage of trained health care workers remains an important
rate-limiting factor in the scale-up of HIV treatment and prevention services,
especially in Africa. The World Health Organization (WHO) has estimated that
Africa bears 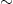 24% of
the global burden of all diseases but has only 3% of the world's healthcare
workforce. Of 57 countries that do not meet basic standards of healthcare
coverage by doctors, nurses, and midwives, 36 are in Sub-Saharan Africa (Kumar,
2007). Significant resources are needed to train doctors and nurses in
resource-poor areas, as well as community healthcare workers to provide care for
HIV/AIDS and other diseases in their home villages and neighborhoods. The model
of community-based care provided by closely supervised “accompagnateurs,”
trained community health workers who use simplified algorithms for diagnosis and
treatment, has been validated in both rich and poor countries and must be better
utilized ([Kim
and Farmer, 2006] and [Mukherjee
et al., 2003]). Operational and clinical research is essential to guide the
formulation of such algorithms and to assess their utility on a larger scale.
24% of
the global burden of all diseases but has only 3% of the world's healthcare
workforce. Of 57 countries that do not meet basic standards of healthcare
coverage by doctors, nurses, and midwives, 36 are in Sub-Saharan Africa (Kumar,
2007). Significant resources are needed to train doctors and nurses in
resource-poor areas, as well as community healthcare workers to provide care for
HIV/AIDS and other diseases in their home villages and neighborhoods. The model
of community-based care provided by closely supervised “accompagnateurs,”
trained community health workers who use simplified algorithms for diagnosis and
treatment, has been validated in both rich and poor countries and must be better
utilized ([Kim
and Farmer, 2006] and [Mukherjee
et al., 2003]). Operational and clinical research is essential to guide the
formulation of such algorithms and to assess their utility on a larger scale.
Infrastructure and personnel for the medical monitoring of ARV therapy are lacking in most poor communities (Koenig et al., 2006). Clearly, limited resources should first be used to scale-up ARV treatment and provide basic support to patients to achieve good treatment adherence and minimize resistance. As rapidly as possible, however, laboratory capacity must be improved to allow the monitoring of virological and immunological outcomes of ARV therapy, as well as toxicities, such that treatment regimens can be optimized (Koenig et al., 2006). Increased funding also is needed to provide services that allow poor people to overcome the social and economic impediments to successful adherence to HIV/AIDS treatment and care. So-called “wrap-around services” frequently include food supplements, transportation to clinics, child care, and housing, as well as care for concomitant diseases ([Kim and Farmer, 2006] and [Mukherjee et al., 2003]).
Striving for universal access to ARVs is a public health and ethical imperative; however, it may be logistically impossible to reach this goal, as six people were newly infected with HIV in 2006 for every person put on treatment (WHO/UNAIDS/UNICEF, 2007). Therefore, HIV prevention activities, hopefully with, but possibly without, a safe and effective HIV/AIDS vaccine will determine the trajectory and burden of the HIV/AIDS pandemic in the years and decades ahead. Today, fewer than one person in five at risk of becoming infected with HIV has access to HIV prevention services, which even when available are confounded by complex cultural and societal issues (Global HIV Prevention Working Group, 2007). For example, a condom is used in only 9% of sex acts involving nonregular partners. Just 10%–12% of adults in the most heavily affected countries in sub-Saharan Africa have been tested and know their HIV serostatus. In developed countries, near-universal provision of ARVs to prevent mother-to-child transmission (PMTCT) has reduced perinatal infection rates to <1%–2% in many cohorts; however, in developing countries, just 11% of HIV-infected pregnant women receive ARV prophylaxis for PMTCT. Prevention services reach a very low proportion of individuals in poor countries at highest risk of HIV infection: just 9% of men who have sex with men, 8% of injection drug users, and <20% of commercial sex workers (Global HIV Prevention Working Group, 2007).
The delivery of proven prevention services is imperative: half of the 60 million HIV infections projected to occur globally between 2005 and 2015 could be averted with comprehensive scale-up of proven prevention strategies, according to a recent estimate (Global HIV Prevention Working Group, 2007). In scaling up prevention services, important lessons can be drawn from common elements of the HIV/AIDS prevention efforts in those countries that have had success in reducing HIV infections. Such factors include the strong support of political leaders and the use of the media to raise HIV awareness; efforts to encourage respect, tolerance, and compassion for HIV-infected people; adequate and sustained funding; the use of evidence-based strategies based on a detailed understanding of the specific dynamics and epidemiology of the epidemic in a particular setting; and the involvement in the prevention effort of diverse sectors, including community groups and religious leaders (Global HIV Prevention Working Group, 2007).
Concomitantly, further research is needed on how best to deliver adult male circumcision (a prevention tool with significant efficacy in clinical trials) and integrate it into existing health services. Research also is needed to prove the efficacy and feasibility of other promising HIV prevention approaches such as topical microbicides, pre-exposure chemoprophylaxis, and an HIV vaccine (Johnston and Fauci, 2007). Each of these interventions could have a significant impact on the course of the HIV pandemic, as part of a multifactorial prevention effort.
A truly comprehensive approach to HIV disease will require a commitment to overcoming the stigma and discrimination frequently associated with HIV infection (UNAIDS, 2006). The medical consequences of stigma and discrimination are serious because HIV-infected people may avoid lifesaving treatment and suffer needlessly. In addition, the psychological toll of isolation and ostracism can be profound, to say nothing of the physical violence to which HIV-infected people are sometimes exposed. Because of stigma, at-risk people may avoid HIV testing altogether, missing opportunities for needed treatment and opportunities to access prevention programs that could help them avoid infecting others.
As we enter the second quarter century of AIDS, we are at a pivotal juncture: there have been substantial scientific advances, yet many infected people are dying needlessly and HIV infection is still spreading. Collectively, we must do more to slow the spread of HIV/AIDS by scaling up the availability of proven treatments and prevention tools. We must eliminate stigma toward and discrimination against people with HIV and refine and validate the efficacy of existing treatment and prevention programs. We must further elucidate the pathogenesis of this complex disease and develop improved treatments and tools for prevention. Perhaps most important, public health workers, policymakers, and activists must bring the benefits of advances in treatment and prevention research to the people who need them most.
Bertozzi et al., 2006 S. Bertozzi, N.S. Padian, J. Wegbreit, L.M. DeMaria, B. Feldman, H. Gayle, J. Gold, R. Grant and T. Isbell, HIV/AIDS prevention and treatment (Chapter 18). In: D.T. Jamison et al., Editors, Disease Control Priorities in Developing Countries (2nd Edition), Oxford University Press, New York (2006) http://www.dcp2.org/pubs/DCP.
Fauci, 2003 A.S. Fauci, Nat. Med. 6 (2003), pp. 839–843. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (65)
Fauci, 2006 A.S. Fauci, Science 313 (2006), p. 409. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (5)
Folkers and Fauci, 2001 G.K. Folkers and A.S. Fauci, JAMA 2864 (2001), pp. 458–461. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (8)
Global HIV Prevention Working Group, 2007 Global HIV Prevention Working Group. (2007). Bringing HIV prevention to scale: an urgent global priority. http://www.kff.org/hivaids/hiv062807pkg.cfm.
Johnston and Fauci, 2007 M.I. Johnston and A.S. Fauci, N. Engl. J. Med. 356 (2007), pp. 2073–2082.
UNAIDS/WHO, 2006 Joint United Nations Programme on HIV/AIDS and World Health Organization (UNAIDS/WHO). (2006). AIDS epidemic update: special report on HIV/AIDS: December 2006. http://www.unaids.org/en/HIV_data/epi2006/.
Kim and Farmer, 2006 J.Y. Kim and P. Farmer, N. Engl. J. Med. 355 (2006), pp. 645–647. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (1)
Koenig et al., 2006 S.P. Koenig, D.R. Kuritzkes, M.S. Hirsch, F. Léandre, J.S. Mukherjee, P.E. Farmer and C. del Rio, BMJ 332 (2006), pp. 602–604. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (4)
Kumar, 2007 P. Kumar, N. Engl. J. Med. 356 (2007), pp. 2564–2567. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (0)
Mukherjee et al., 2003 J.S. Mukherjee, P.E. Farmer, D. Niyizonkiza, L. McCorkle, C. Vanderwarker, P. Teixeira and J.Y. Kim, BMJ 327 (2003), pp. 1104–1106. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (24)
Siegel and Gulick, 2007 L. Siegel and R.M. Gulick, Curr. Infect. Dis. Rep. 9 (2007), pp. 243–251. Full Text via CrossRef
WHO/UNAIDS/UNICEF, 2007 World Health Organization, UNAIDS, UNICEF (WHO/UNAIDS/UNICEF). (2007). Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress reports, April 2007. http://www.who.int/hiv/mediacentre/en.
UNAIDS, 2006 UNAIDS. (2006). 2006 report on the global AIDS epidemic—a UNAIDS 10th anniversary special edition. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
United Nations Population Division, 2006 United Nations Population Division. (2006). World population prospects: the 2006 revision (highlights). http://www.un.org/esa/population/publications/wpp2006/wpp2006.htm.
 Volume 131, Issue 3, 2 November 2007, Pages 429-432 |
| This article originally appeared in Cell and is presented with permission from that publication. |