Updated 6/08
Facts About Rare Muscular Dystrophies
(Congenital, Distal, Emery-Dreifuss and Oculopharyngeal)
For the Spanish version of this publication, click here: En Español |
Dear Friends:
 |
| Mike Neufeldt |
When I was about a year old, my parents noticed something odd about the way I walked. After many tests it was determined that I had a neuromuscular disease.
It took us several years to get a definite diagnosis of Emery-Dreifuss muscular dystrophy.
If you’ve recently found out you have a rare form of muscular dystrophy, you understand what my family went through. The rarity of Emery-Dreifuss, congenital, distal and oculopharyngeal muscular dystrophies makes it very important that you get all the information you can about your disorder. This booklet will help you get started.
Learning that you or your child has a rare form of MD can be frightening and confusing. My parents wondered why I had this disease; we had no history of it in our family. But, as this booklet explains, each type of MD is caused by an extremely uncommon genetic defect that people often don’t even know they have. You can be sure your disorder wasn’t caused by anything you or your parents did, and you didn’t catch it from anyone.
My family had to make many adjustments because of MD. But we were lucky. Along with great, caring doctors, we had the Muscular Dystrophy Association to help us. From MDA, my folks got the support and information they needed, as well as help with equipment and other services. I was honored to serve as MDA’s National Goodwill Ambassador in 1987-88.
I’m grateful to my parents for not hiding things from me and for letting me be a part of discussions with doctors from an early age. Understanding my disease helped prepare me to manage my medical care as an adult. Having information about my MD also enabled me to enjoy a typical childhood, with sports, Cub Scouts and many friends. In high school I kept up high grades, was a team statistician and had a part-time job.
I graduated from college with a degree in communications, and I work in interactive communications at Harley-Davidson Motor Co. in Milwaukee. I use a power wheelchair and part-time respiratory assistance.
I’m telling you about myself so you’ll see that people with rare MDs can have fulfilling, happy lives. It isn’t easy to live with muscles that grow weaker over time, but you don’t have to let MD keep you from pursuing an education, career, family, travel — anything you want.
People with disabilities have more opportunities today than ever before to develop and use their abilities. Federal law guarantees us a public education, equal employment opportunity and access to public places. Computers and technology help us to move around, write, work and drive.
“MDA Is Here to Help You” tells of the Association’s invaluable services. MDA is also the world leader in research on neuromuscular diseases, and its scientists have made many exciting discoveries about all forms of MD in recent years.
This booklet will give you the basic facts about these four forms of MD, and MDA will help you answer all your questions as they arise. As you face the challenges ahead, please be assured that we’re making rapid progress toward better treatments and a cure. And remember, you’re not alone.
Mike Neufeldt
Milwaukee
MDA National Task Force on Public Awareness
What is Muscular Dystrophy?
The muscular dystrophies are a group of genetic diseases that cause weakness and muscle wasting, primarily in the skeletal or voluntary muscles (those we control such as the muscles of the arms and legs). The four types of muscular dystrophy (MD) described in this booklet — congenital muscular dystrophy (CMD), distal muscular dystrophy (DD), Emery-Dreifuss muscular dystrophy (EDMD) and oculopharyngeal muscular dystrophy (OPMD) — are among the rarer forms of muscular dystrophy. Because they’re less common, they can be difficult to diagnose, and many questions remain to be answered about their symptoms and progression.
The congenital muscular dystrophies and the distal muscular dystrophies (sometimes called distal myopathies) are both groups of muscle disorders. Emery-Dreifuss and oculopharyngeal each appears to be a single form of muscular dystrophy in terms of symptoms (although EDMD and OPMD can have more than one genetic cause).
Most people with muscular dystrophy experience some degree of muscle weakness during their lifetimes, but each of the four disorders described in this booklet affects different muscle groups and may have different accompanying symptoms. Because muscle weakness usually progresses over time in the muscular dystrophies, lifestyle changes, assistive devices and occupational therapy may be needed to help a person adapt to new situations.
What causes muscular dystrophy?
All the forms of muscular dystrophy are inherited — that is, they’re caused by mutations (changes) in a person’s genes. Our genes are made of DNA and they reside in our chromosomes. Each gene contains the “recipe” for a different protein and its variations, and these proteins are necessary for our bodies to function correctly.
When a gene has a mutation, it may make a defective protein or none at all. Most commonly, missing or defective proteins in the muscles prevent muscle cells from working properly, leading to symptoms of muscular dystrophy, including muscle weakness and wasting over time.
Muscles Up Close
Muscles are made up of bundles of fibers (cells). Groups of proteins along the membrane surrounding each fiber and within the cell help to keep muscle cells working properly. When one of these proteins is absent or inadequate (because a gene fails to make it properly), the result can be a form of muscular dystrophy. Absence of or defects in different proteins are among the causes of different types of muscular dystrophy.
Various forms of congenital muscular dystrophy arise from defects in proteins in or outside the membrane of the muscle cell (fukutin, integrin), or in the extracellular matrix, which attaches to the membrane (merosin or laminin-alpha-2.) Another membrane protein, dysferlin, is involved in distal muscular dystrophy.
The absence of some protein functions in the cell’s nucleus leads to Emery-Dreifuss muscular dystrophy (emerin, lamin A, lamin C) or oculopharyngeal muscular dystrophy (PABPN1).
(The absence of other vital muscle proteins (not identified) leads to muscular dystrophies not covered in this booklet, such as Duchenne MD.)
 |
What happens to someone with muscular dystrophy?
Most forms of muscular dystrophy are progressive, and they tend to worsen with time. However, age of onset and rate of progression can vary widely from one disorder to the next. Some, but not all, of these disorders can affect life expectancy. In many cases, advancing knowledge allows for treatment of the symptoms that are most likely to decrease life expectancy.
In most cases of muscular dystrophy, muscle mass in the affected regions may become visibly wasted (decreased in size), and the arms, legs or trunk may become so weak they eventually can’t move. Some forms of muscular dystrophy are accompanied by contractures, or stiff joints, and some are accompanied by scoliosis, or spinal curvature.
Forms of muscular dystrophy that affect the muscles used for swallowing may require that precautions be taken when eating or drinking so that food isn’t aspirated into the lungs. Although most muscular dystrophies don’t affect the brain, some are accompanied by brain changes that cause learning disabilities that range from slight to severe.
Finally, some forms of muscular dystrophy also affect the heart, and special precautions must be taken to monitor heart function. Each disorder has its own special areas of concern.
What tests are used to diagnose muscular dystrophy?
In diagnosing any form of muscular dystrophy, a doctor usually begins by taking a patient and family history and performing a physical examination. Much can be learned from these, including the pattern of weakness. The history and physical go a long way toward making the diagnosis, even before any complicated diagnostic tests are done.
The doctor also wants to determine whether the patient’s weakness results from a problem in the muscles themselves or in the nerves that control them. Problems with muscle-controlling nerves, or motor nerves, originating in the spinal cord and reaching out to all the muscles, can cause weakness that looks like a muscle problem but really isn’t.
Usually, the origin of the weakness can be pinpointed by a physical exam. Occasionally, special tests called nerve conduction studies and electromyography (EMG) are done. In these tests, electricity and very fine pins are used to stimulate and assess the muscles or nerves individually to see where the problem lies. Electromyography is uncomfortable but not usually very painful.
Early in the diagnostic process doctors often order a special blood test called a CK level. CK stands for creatine kinase, an enzyme that leaks out of damaged muscle. When elevated CK levels are found in a blood sample, it usually means muscle is being destroyed by some abnormal process, such as a muscular dystrophy or inflammation. Therefore, a high CK level often suggests that the muscles themselves are the likely cause of the weakness, but it doesn’t tell exactly what the muscle disorder might be.
To determine which disorder is causing CK elevation, a doctor may order a muscle biopsy, the surgical removal of a small sample of muscle from the patient. By examining this sample, doctors can tell a great deal about what’s actually happening inside the muscles. Modern techniques can use the biopsy to distinguish muscular dystrophies from infections, inflammatory disorders and other problems.
Other tests on the biopsy sample can provide information about which muscle proteins are present in the muscle cells, and whether they’re present in the normal amounts and in the right locations. This can tell the doctor and patient what’s wrong with the cells’ proteins and provide likely candidates as to which genes are responsible for the problem. The correlation between missing proteins on the muscle biopsy and genetic flaws isn’t perfect, however. An MDA clinic physician can help you understand these results.
Genetic (DNA) tests, using a blood sample, can analyze the person’s genes for particular defects that cause the rare muscular dystrophies, help predict the likely course of a disease and help families assess the risk of passing on the disease to the next generation. DNA tests for most of the diseases in this booklet are commercially available in the United States, but, as of 2008, testing for some of the rarer disorders is found only through research studies. DNA testing is likely to become more widely available in the next few years.
Does it Run in the Family?
On being told they have a genetic disorder such as muscular dystrophy, bewildered patients often ask, “But it doesn’t run in the family, so how could it be genetic?”
Muscular dystrophy can run in a family, even if only one person in the biological family has it. This is because of the ways in which genetic diseases are inherited.
Each form of muscular dystrophy follows one of three patterns of inheritance: recessive, dominant or X-linked. In brief, if a disease is recessive, two copies of the defective gene (one from each parent) are required to produce the disease. Each parent would be a carrier of the gene flaw, but wouldn’t usually have the disease.
If a disease is dominant, then only one copy of the genetic defect is needed to cause the disease. Anyone with the gene flaw will have disease symptoms and can pass the disorder to children.
If a disease is X-linked, it’s passed from mother to son, while daughters can be carriers but don’t generally get the disease.
Many times MD appears to have occurred “out of the blue,” but in reality, one or both parents may be carriers, silently harboring the genetic mutation (a flaw in the gene). Many parents have no idea they’re carriers of a disease until they have a child who has the disease.
In rare cases, muscular dystrophy actually can occur “out of the blue” when a new mutation appears with a baby’s conception, though neither parent carries the gene flaw. These are called spontaneous mutations, and, after they occur, they can be passed on to the next generation, thereby introducing the gene for a specific MD into the family.
The risk of passing on a form of muscular dystrophy to your children depends on many circumstances, including exactly which type of MD has been diagnosed. A good way to find out more about these risks is to talk to your MDA clinic physician or ask to see the genetic counselor at the MDA clinic. You also can see MDA’s pamphlet “Facts About Genetics and Neuromuscular Diseases.” |
What can be done to treat muscular dystrophy?
There are currently no cures for any form of muscular dystrophy, but there are many therapies designed to help deal with common symptoms of the disease. For instance, contractures may be helped by physical therapy and sometimes tendon-release surgery, while scoliosis may respond to bracing or surgery, and heart problems may respond to medication or an implanted pacemaker. (Physical and occupational therapies can be arranged through your child’s school or through your MDA clinic.)
Many people with these forms of muscular dystrophy live very full lives. Your MDA clinic director will help you plan the best strategy for coping with your or your child’s specific needs.
top 
Congenital Muscular Dystrophy (CMD)
The term congenital muscular dystrophy (CMD) is actually the name for a group of muscular dystrophies that are united by the fact that muscle weakness begins in infancy or very early childhood (typically before age 2). Congenital diseases are those in which the symptoms are present at or soon after birth.
A diagnosis of CMD can be confusing because for many years the term was used as a “catchall” name to describe conditions that looked like other muscular dystrophies, but started much earlier or followed different patterns of inheritance. In recent years doctors have agreed that there are several categories of “true” CMD, caused by specific gene mutations, and they’re distinct from other muscular dystrophies. It’s possible that some people who received diagnoses of CMD many years ago may actually have some other known form of muscular dystrophy with an unusually early onset.
Although children with CMD can have different associated symptoms, degrees of severity and rates of progression, most exhibit some progressive muscle weakness. This weakness, usually first identified as hypotonia, or lack of muscle tone, can make an infant seem “floppy.” Later, infants and toddlers may be slow to meet motor milestones such as rolling over, sitting up or walking, or may not meet some milestones at all.
Some of the rarer forms of CMD are also accompanied by significant learning disabilities, or mental retardation.
What causes CMD?
The CMDs are caused by genetic defects that affect important muscle proteins. Most forms of CMD are inherited in an autosomal recessive pattern, but at least one form appears to follow a dominant pattern of inheritance. (For more about inheritance patterns, see “Does It Run in the Family?”.)
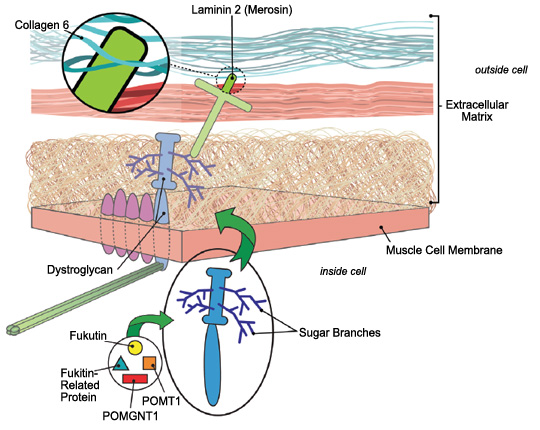 |
| Most forms of congenital muscular dystrophy stem from loss of firm connections between muscle fibers and their surroundings (extracellular matrix). |
It isn’t known why the CMDs cause muscle weakness earlier than other types of muscular dystrophy. One possibility is that the muscle proteins affected in CMD are required early in the development of an infant’s muscle, while muscle proteins linked to other muscular dystrophies don’t become important until the muscles begin to get a lot of use as a child grows.
It’s important to note that just because the muscle weakness in CMD starts earlier, CMD isn’t automatically more severe than other forms of muscular dystrophy. The degree and rate of progression of muscle weakness varies with different forms of CMD and from one child to the next.
In the mid-1990s, researchers found that a deficiency of a protein then called merosin and now more often called laminin 2 was the underlying cause of at least some cases of CMD. Merosin normally anchors muscle cells to a structure that encases them (like the skin on a hot dog) called the basal lamina.
Doctors began to classify CMD as either “merosin-deficient” or “merosin-positive.” The gene for merosin is on chromosome 6.
Then, in 1998, researchers identified mutations in the gene for integrin as another cause of CMD. Integrin, which surrounds and supports each muscle fiber, connects laminin 2 with proteins inside the cells.
As the 20th century ended, researchers began to suspect that Ullrich’s disease, now known as Ullrich CMD, was caused by a lack of collagen 6, a ropelike protein located in the area where laminin 2 is found.
Collagen 6, which helps support the muscle fiber, probably affects muscle cells via its connection to laminin 2. Laminin 2, in turn, connects to muscle cells via either of two other proteins: integrin or dystroglycan.
Dystroglycan links the outer surface of muscle cells with structures outside them via branches, made of sugar molecules, that protrude from its surface and stick to laminin.
The branch structure explains why mutations in diverse genes all appear to cause CMD. Each of these proteins contributes in a different way to the process of “sugar coating” (glycosylating) dystroglycan. Several forms of CMD that affect not only the muscles but the eyes and brain — Fukuyama CMD (seen mainly in Japan), muscle-eye-brain disease and Walker-Warburg syndrome — arise from defects in these glycosylation proteins.
The illustration shows the physical relationships among these proteins.
In 2001, researchers identified mutations in the gene for selenoprotein N1 as a cause of CMD with a rigid spine and sometimes frozen joints at the elbows, hips, ankles or knees. This protein is believed to play a role in muscle development early in life and doesn’t appear to be part of the linked protein cluster shown below.
For more about the causes of specific CMDs and their inheritance patterns, see “Classification of CMDs”.
What are the types of CMD?
Merosin-deficient CMD
 |
| This 4-year-old has merosin-deficient CMD. |
Children with merosin-deficient CMD lack all or part of the muscle protein merosin, or laminin 2. The disease commonly makes itself known when a child fails to learn to walk. The degree of muscle weakness can range from severe (never walking) to mild (walking at 2 to 3 years), and depends on how much merosin protein a child has.
This form of CMD progresses very slowly or, in some cases, not at all. Special problems include contractures, difficulty breathing and seizures (in 20 percent of cases). Intelligence is usually normal, but learning disabilities have been documented.
A distinctive diagnostic feature of this type of CMD is found by magnetic resonance imaging (MRI). These brain images show changes in the white matter, which consists of nerve fibers that carry messages from the brain to the spinal cord. Despite the appearance on the MRI, those with merosin-negative CMD have few signs of brain impairment in everyday life.
Ullrich CMD
 |
| This teen with Ullrich CMD plays the saxophone and guitar, and is in honors classes. |
Clinical characteristics of Ullrich CMD (or Ullrich’s disease) include hypotonia (loss of muscle tone), hyperlaxity (loose joints) in the hands and feet, and multiple joint contractures at birth, with rigidity of the spine. The course is slowly progressive, causing muscle weakness and wasting.
Intelligence is normal. Children with Ullrich’s disease may develop respiratory failure during sleep in the first decade of life.
Bethlem myopathy
This rare form of CMD is, like Ullrich’s, caused by a lack of collagen, but it has a less severe course. It can first appear at any age, and features restricted joint mobility, including finger contractures. (It’s called a myopathy, which means “muscle disease,” a broader term than dystrophy, which implies progressive degeneration.)
Integrin-deficient CMD
Children with integrin deficiency have hypotonia (lack of muscle tone) and weakness early in infancy associated with delayed milestones. Children usually don’t walk until age 2 to 3.
Fukuyama CMD (FCMD)
 |
| CMD can cause contractures in the wrists, ankles and other joints. |
Fukuyama congenital muscular dystrophy is seen almost exclusively in those of Japanese descent. The disorder has been linked to a defect in a gene called fukutin, and the most common mutation is thought to have arisen in a single Japanese ancestor many years ago. Evidence suggests that fukutin participates in the sugar coating of dystroglycan.
Muscle weakness in FCMD ranges from severe to mild, and people with the mildest cases are able to walk with assistance. Extensive brain abnormalities are usually accompanied by severe mental retardation, epilepsy, visual loss and reduced life expectancy (about 11 to 16 years of age).
Muscle-eye-brain disease (MEB)
This rare form of CMD first described in Finland shares features with FCMD. Generally it’s milder, with survival ranging from early childhood to the seventh decade. It’s accompanied by delayed motor milestones, severe mental retardation and vision problems.
 |
| Physical therapy is important in maintaining range of motion and reducing contractures. |
Walker-Warburg syndrome (WWS)
This very rare form of CMD is similar to but more severe than MEB. (Experts consider MEB and WWS to be a spectrum of disorders.) People with this disorder have hypotonia and seizures. Severe mental retardation and multiple vision problems are encountered. The disorder is usually lethal in infancy.
CMD with rigid spine syndrome
This form of CMD is characterized by onset before 1 year of age with prominent neck weakness and poor head control. After some initial improvement, children gradually develop (by 3 to 7 years) stiffness or rigidity of the spine. To a lesser extent contractures of limb muscles are seen.
 |
| Specially adapted computers can help children with vision problems. |
By the teens, the respiratory muscles are affected, while limb muscle strength is less affected. Intellectual function is normal.
Problems and solutions in congenital muscular dystrophies
Contractures
Stiff or “frozen” joints (contractures) can be present at birth or develop as muscles weaken, but regular physical therapy designed to maintain range of motion at the joints can help combat this problem.
Scoliosis
Weak trunk muscles can lead to curvature of the spine, or scoliosis, which, in turn, can limit mobility and interfere with breathing. Corrective surgery may eventually be required.
Muscle weakness
Leg braces or a wheelchair may eventually be needed to help with mobility. An occupational therapist can help those with CMD find the best ways to perform day-to-day functions, often through use of assistive devices.
Respiratory insufficiency
Advanced or severe weakness of the respiratory muscles (the diaphragm and rib cage muscles) may interfere with breathing. Symptoms of respiratory insufficiency include morning headaches, fatigue, sleeplessness, weakened or softened voice, and coughing. There are many options available to help with this problem, ranging from noninvasive nighttime ventilation to a tracheostomy.
Learning disabilities
Some children with CMD may have significant learning disabilities or mental retardation. Special education programs, begun as early as possible, can help a child maximize learning potential.
Seizures and vision problems
Specialists can address these problems with a variety of therapies.
top 
Classification of Congenital Muscular Dystrophies |
Type of CMD |
Cause |
Inheritance Pattern |
| Merosin-deficient CMD |
lack of merosin (laminin 2) or other defect leading to merosin deficiency |
Chromosome 6 gene. Other genes |
| Ullrich CMD |
abnormalities in collagen 6 |
Chromosome 2 or 21 genes, recessive or dominant |
| Bethlem myopathy |
abnormalities in collagen 6 |
Chromosome 2 or 21 genes, dominant |
| Integrin-deficient CMD |
lack of integrin alpha 7 |
Chromosome 12 gene, recessive |
| Fukuyama CMD (FCMD) |
lack of fukutin |
Chromosome 9 gene, recessive |
| Muscle-eye-brain disease (MEB) |
lack of POMGnT1, fukutin or fukutinrelated protein |
Chromosome 1, 9 or 19 genes, recessive |
| Walker-Warburg syndrome (WWS) |
lack of POMT1, POMT2, fukutin or fukutin related protein |
Chromosome 9, 14 or 19 genes, recessive |
| CMD with rigid spine syndrome |
lack of selenoprotein N1 |
Chromosome 1 gene, recessive |
See illustration. |
Distal Muscular Dystrophy (DD)
First described in 1902, distal muscular dystrophy (DD), or distal myopathy, is the name of a group of disorders that primarily affect distal muscles, those farthest away from the hips and shoulders such as muscles in the hands, feet, lower arms or lower legs. Although muscle weakness is usually first detected in the distal muscles, with time, other muscle groups may become affected as well. Intellect isn’t affected in these diseases.
What causes DD?
The DDs are caused by many different genetic defects, not all of which are yet known. Also, some of the DDs have been given different names based on various symptoms but may actually be caused by defects in the same gene.
Your own form of DD may or may not fit into one of these categories. Many of these diseases can vary from one person to the next, and in some cases, researchers are still in the process of sorting out what symptoms are linked to a particular genetic defect.
What are the types of distal muscular dystrophy?
Welander’s distal myopathy
This form of distal muscular dystrophy usually has an onset between 40 and 50 years of age. Upper extremities tend to be affected first, then lower ones. The degree of muscle weakness involved can range from mild to severe. The cause remains unknown.
Finnish (tibial) distal myopathy
 |
| Lower leg braces can support muscles weakened by distal MD. |
Finnish muscular dystrophy features weakness starting after age 40 in the lower extremities (particularly the muscles over the tibia, a bone in the lower leg) and progressing slowly to the upper extremities and trunk muscles. Cardiac problems can be a feature. This distal myopathy results from mutations in the protein titin, which plays a role in muscle fiber structure and force generation.
Finnish muscular dystrophy (also called tibial MD) can be severe or benign, and typically affects only people of Finnish descent. Those with only one defective gene experience mild weakness of the tibial leg muscles (front of the calf) sometime after age 40. Those with two defective genes have progressive weakness starting in childhood and may lose the ability to walk by age 30.
Miyoshi distal myopathy
This disorder involves weakness that begins in the lower extremities, especially in the calf muscles. It can progress to other muscles as well. Symptoms usually begin between 15 and 30 years of age.
The genetic defects that cause Miyoshi myopathy are in the gene for the dysferlin protein. Defects in the dysferlin gene also can cause limb-girdle muscular dystrophy 2B, which results in muscle weakness in and around the hips and shoulders. People with the same genetic defect in their dysferlin genes can have either disease, and it isn’t known what determines which pattern of symptoms a person gets.
Nonaka distal myopathy
 |
| Inclusion-body myositis first affects the lower legs and thighs. |
Usually found in families of Japanese descent, this DD has symptoms that begin between ages 20 and 40. The anterior lower leg muscles (those in the front of the leg) are typically affected first, but the disease may progress to affect upper arm and leg muscles and neck muscles. The quadriceps muscles (in the thigh) tend to remain strong.
The disease is caused by defects in the GNE gene, the same gene that underlies one form of hereditary inclusionbody myositis (HIBM2). (This condition also is called inclusion-body myopathy.)
The GNE protein that comes from this gene modifies compounds on cell surfaces in a way that’s needed for cells to signal each other and adhere to each other.
Gowers-Laing distal myopathy
This disorder has its onset from childhood to 25 years of age. Weakness is first seen in the leg and neck muscles, and progresses slowly to include upper leg muscles, hands and more neck muscles.
Gowers-Laing distal myopathy results from mutations in the MYH7 gene, which instructs for myosin heavy chain 7, a protein that participates in muscle contraction.
Hereditary inclusion-body myositis (myopathy) type 1 (HIBM1)
HIBM1 usually begins between the ages of 25 and 40, first affecting the muscles that lift the front of the foot and the thigh muscles. Other muscles can be affected later. Under the microscope, muscle cells show inclusion bodies, which are abnormal clumps of cellular material; and vacuoles, which are cellular bubbles. The cause is unknown.
Distal myopathy with vocal cord and pharyngeal weakness
This disorder has been linked to chromosome 5 in the same region as the gene that’s defective in limb-girdle MD type 1A. Symptoms first appear between about 35 and 60 years of age and include weakness of the hands, legs or voice. Difficulty in swallowing may be a feature.
ZASP-related myopathy
This disorder, identified in 2005, involves abnormalities in a protein that’s part of the molecular apparatus that allows muscles to contract. Weakness is generally in the distal and proximal (close to the center of the body) muscles, although distal weakness predominates. The heart also can be affected. In the 11 patients identified in 2005, the age range for disease onset was 44 to 73.
 |
| An occupational therapist can show those with DD how to perform activities in spite of weak arm muscles. |
Problems and solutions in DD
Lower leg and foot weakness
Weakness of the lower leg and foot muscles can make walking difficult. In some cases, an ankle-foot orthosis (AFO), a brace worn over the shoe and lower leg, can help.
AFOs are especially useful when muscles in the front of the lower leg aren’t strong enough to pick up the front of the foot during walking. In this condition, known as foot drop, an AFO can prevent the foot from flopping down and tripping the person.
Forearm and hand weakness
Your MDA clinic can refer you to an occupational therapist who can help you get the most out of your hand and forearm muscles in performing day-to-day activities. Often, the therapist can recommend devices that may improve grip strength or help support your arms for using a keyboard or eating.
Classification of Distal Muscular Dystrophies |
Type of DD |
Cause |
Inheritance Pattern |
| Welander’s distal myopathy |
abnormalities in chromosome 2 gene |
dominant |
| Finnish (tibial) distal myopathy |
titin abnormalities |
dominant |
| Miyoshi distal myopathy |
dysferlin abnormalities |
recessive |
| Nonaka distal myopathy; also called hereditary inclusion-body myositis type 2 (HIBM2) |
GNE abnormalities |
recessive |
| Gowers-Laing distal myopathy |
MYH7 abnormalities |
dominant |
| Hereditary inclusion-body myositis type 1 (HIBM1) |
unknown |
dominant |
| Distal myopathy with vocal cord and pharyngeal weakness |
abnormalities in chromosome 5 gene |
dominant |
| ZASP-related myopathy |
ZASP abnormalities |
dominant |
Source: Washington University Neuromuscular Home Page, May 2006 |
top 
Emery-Dreifuss Muscular Dystrophy (EDMD)
Emery-Dreifuss muscular dystrophy is characterized by wasting and weakness of the muscles that make up the shoulders and upper arms and those of the calf muscles of the legs. Another prominent aspect of this disease is the appearance of contractures (stiff joints) in the elbows, neck and heels very early in the course of the disease. Finally, and very importantly, a type of heart problem called a conduction block is a common feature of EDMD and requires monitoring.
How is EDMD inherited?
The most common form of EDMD is inherited in an X-linked pattern, but EDMD also can be inherited in a dominant fashion and, very rarely, in a recessive fashion. Although dominant, recessive and X-linked EDMD follow different patterns of inheritance, their symptoms are almost indistinguishable.
What causes EDMD?
Researchers recently have identified the genes that, when defective, lead to the forms of EDMD. We now know that the gene that’s defective in X-linked EDMD makes a small protein called emerin, which normally is located in the membrane that surrounds each cell’s nucleus (the compartment in a cell’s center that contains the chromosomes).
It isn’t yet understood how the loss of emerin from the nuclear membrane in X-linked EDMD leads to the symptoms of muscular dystrophy. Some researchers think this lack of emerin interferes with the reorganization of the nuclear membrane after a cell has divided, leading to weak or dying cells. Along these same lines, the gene that’s been found defective in both the autosomal and recessive forms of EDMD contains the instructions for two closely related proteins called lamin A and lamin C that also are associated with the nuclear membrane of cells.
Again, it isn’t yet known how changes in lamins A and C lead to the symptoms of muscular dystrophy, but some research suggests that the nuclear membrane may become destabilized when the lamin proteins are abnormal. This could lead to muscle breakdown.
Another question that remains to be answered is why the symptoms of EDMD are restricted primarily to the skeletal (voluntary) muscles and heart muscle, given that the emerin and lamin proteins are found in most tissues of the body.
What happens to someone with EDMD?
 |
| EDMD leads to difficulty bending the elbows, muscle weakness and cardiac problems. |
The symptoms of EDMD usually become apparent by 10 years of age, but the disorder tends to progress slowly. Early signs include “toe-walking” because of stiff Achilles’ tendons in the heels, and difficulty bending the elbows. Later the signs of muscle weakness become more prominent but are still generally considered mild. Usually cardiac problems are detectable by age 20, but they can occur at earlier stages in the disease as well. Intellect isn’t affected.
The contractures that occur early in EDMD may make arm, neck, heel and spine movements difficult; however, the progression of muscle weakness seems to occur very slowly in EDMD and may not become a source of difficulty until later in life.
Cardiac problems can be life-threatening and may require the insertion of a pacemaker or treatment with medications.
Some women who are genetic carriers for X-linked EDMD also may be at risk for cardiac problems and this risk may increase with age. (X-linked EDMD carriers don’t tend to have muscle weakness or contractures.)
Problems and solutions in EDMD
Contractures
 |
| It’s important for people with EDMD to monitor their heart function. |
Contractures develop early in EDMD and can worsen even if muscle strength doesn’t change. Preventing contractures is difficult, but maintaining range of motion with physical therapy may help to slow their development. Surgical release of contractures is challenging because of their tendency to recur.
Cardiac conduction block
This form of heart problem occurs when the rhythm of the heartbeat is disrupted because the electrical impulses don’t communicate properly between the heart’s upper and lower chambers. Conduction block can lead to sudden cardiac arrest. By age 30, almost all those with EDMD will have some form of detectable cardiac involvement.
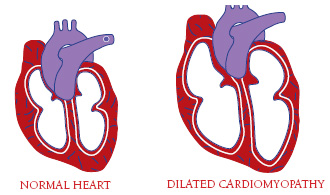 |
| A floppy, dilated heart can’t pump effectively. |
Fortunately, this problem is fairly easy to detect with an electrocardiogram, and the insertion of a pacemaker can be lifesaving. Anyone given a diagnosis of EDMD should be monitored regularly for signs of cardiac conduction block.
Cardiomyopathy
In addition to cardiac conduction abnormalities, many people with EDMD ultimately develop dilated cardiomyopathy, an impairment in the heart muscle’s ability to pump blood around the body because it’s thinned out and floppy (dilated). Medications can help when the heart is impaired in this way.
Oculopharyngeal Muscular Dystrophy (OPMD)
Oculopharyngeal muscular dystrophy (OPMD) is characterized by weakness of the muscles that control the eyelids (leading to droopy eyelids, a condition also known as ptosis), and by weakness of the facial muscles and pharyngeal muscles (those in the throat used for swallowing). It also affects limb muscles. Symptoms of the disease usually don’t begin until the mid-40s or 50s, but can occur earlier.
OPMD is usually inherited as a dominant disease, but rare cases may show a recessive pattern of inheritance. When muscle tissue from a person with OPMD is examined under a high-powered microscope, clumps of proteins called inclusions are seen in the muscle cell nuclei (the cellular compartments that contain the chromosomes), and bubblelike structures (vacuoles) appear in the muscle cells.
The disease is most common in French-Canadian families or families of French-Canadian descent. There’s also a high incidence of OPMD among Hispanic residents of northern New Mexico.
OPMD also can affect people who aren’t of French-Canadian or Hispanic background.
What causes OPMD?
The gene that’s defective in OPMD was discovered in 1998. It carries instructions for a polyadenylate binding protein (PABPN1) that’s normally present in the cell nucleus. Researchers suspect that in OPMD, the presence of extra amino acids in the protein made from a defective PABPN1 gene causes the PABPN1 protein to clump together in the muscle cell nuclei, perhaps interfering with cell function. The disease often can be diagnosed with a DNA test for the most common PABPN1 mutation.
 |
| When swallowing muscles don’t function normally, food and liquids can be routed to the airway and lungs instead of to the esophagus and stomach. |
What happens to someone with OPMD?
 |
| Droopy eyelids are a common characteristic of OPMD. |
A person with OPMD may first notice drooping eyelids that gradually lead to tipping the head backward to see properly. Alternatively, some people might first notice that they tend to choke frequently and may have other problems related to difficulty swallowing (called dysphagia). Most people eventually develop some degree of both ptosis and dysphagia.
Eventual weakness of the muscles in the face and limbs is common. For instance, many people with OPMD report problems with kneeling, bending, squatting, walking and climbing stairs. Double vision and a “breathy” quality of the voice also may occur.
Currently, there’s no cure for OPMD, but many people with the disease find that treating symptoms as they occur is beneficial.
Problems and solutions in OPMD
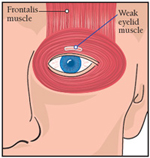 |
| The purpose of a frontalis sling operation is to allow the frontalis muscle, which normally maintains its strength in OPMD, to open the eye. |
Dysphagia
Difficulty swallowing, or dysphagia, can cause a person to aspirate food or liquid into the lungs, which in turn may lead to a serious problem called aspiration pneumonia. If you find that you’re choking frequently while eating or drinking, you may need to have your swallowing abilities evaluated by a professional. There are a number of techniques that may help treat dysphagia, ranging from holding the head in different positions to changing the consistency of foods and liquids. Commercial thickeners may give liquids a more manageable consistency. In advanced cases, a nonsurgical procedure called throat stretching or a surgical procedure called a cricopharyngeal myotomy may be warranted. Tube feeding is another option for advanced cases.
Your MDA clinic will refer you to a speech-language pathologist (SLP) or an otolaryngologist (ear, nose and throat doctor) as needed.
 |
 |
| For news about CMD, DD, EDMD and OPMD see “What’s New” and “Clinical Trials” on the MDA Web site, www.mda.org. |
Ptosis
Droopy eyelids, or ptosis, can significantly impair vision and may lead to social awkwardness.
This problem can be resolved with a type of surgery called a frontalis sling performed by an oculoplastic surgeon. Or, for a “low-tech” solution, some people use attachments to glasses that hold the eyes open. These are called eyelid crutches or ptosis crutches.
Limb weakness
Trouble with picking up the feet when walking can lead to stumbling and falls. Leg braces, a cane or walker can help. Eventually, those with OPMD may need to use a wheelchair to make mobility more convenient.
Upper arm and shoulder weakness that limits function can be addressed with adaptive techniques through occupational therapy.
top 
MDA’s Search for Treatments & Cures
Ever since ground-breaking research in 1986 allowed MDA-sponsored scientist Louis Kunkel to identify the gene involved in Duchenne muscular dystrophy, MDA researchers have forged ahead to isolate and characterize genes involved in almost all the neuromuscular disorders covered in MDA’s program. These include many of the gene defects responsible for the disorders described in this booklet. These discoveries have enabled scientists to understand variations among different forms of the diseases and have helped doctors to provide more accurate diagnosis.
| MDA's Web site is constantly updated with the latest information about the neuromuscular diseases in its program. See the latest research news. |
Now that this essential first step is almost accomplished in its entirety, MDA is exploring ways to correct muscle problems caused by the different gene defects. Areas of especially active research include:
- Gene therapy: A mechanism for supplementing defective genes with healthy genes in the tissues affected by neuromuscular disease
- Gene silencing: Turning off genetic instructions that cause the production of toxic proteins
- Cell therapy: Transplanting new muscle cells, using stem cells or immature muscle cells from a donor or genetically corrected cells from the patient’s own body
MDA is committed to finding cures for all of the neuromuscular disorders in its program and will continue to fund cutting-edge research to attain this end. |
MDA is Here to Help You
The Muscular Dystrophy Association offers a vast array of services to help you and your family deal with CMD, DD, EDMD or OPMD. The staff at your local MDA office is there to assist you in many ways, with services including:
- a nationwide network of hospital-affiliated clinics staffed by top neuromuscular disease specialists
- MDA summer camps for kids with neuromusulcar diseases
- professionally facilitated support groups for those affected, parents, spouses or other caregivers
- assistance with purchase and repair of wheelchairs, leg braces and augmentative communication devices
- evaluations for physical, occupational and respiratory therapy
- flu shots to help protect the respiratory system
- equipment loan closets
MDA’s public health education program helps you to stay abreast of research news, medical findings and disability information related to CMD, DD, EDMD, and OPMD. MDA’s Web site at www.mda.org offers thousands of pages of valuable information, including news and online chats.
MDA publishes many brochures and booklets about living with neuromuscular diseases, available in Spanish and English. Everyone registered with MDA also receives Quest, MDA’s quarterly national magazine.
If you have any questions about CMD, DD, EDMD or OPMD, someone at MDA will help you find the answers.
top 
Facts About Rare Muscular Dystrophies
|
 Back to Disease Booklets
Back to Disease Booklets
|