Influenza and Antiviral Drugs
Health care providers: monitor influenza activity in your area and review CDC's Interim Antiviral Guidance.
Although a yearly vaccine is the best way to protect against the flu, antiviral drugs are an important second line of defense. There are four prescription antiviral drugs approved for use in the United States to treat and/or prevent influenza virus infection: the adamantanes (amantadine and rimantadine) and the neuraminidase inhibitors (oseltamivir and zanamivir).
However, the constantly changing nature of influenza viruses and differences in circulation can make the selection of which antiviral drug to use challenging. This is because small changes in influenza viruses can make certain types and subtypes of influenza viruses resistant to one or more of the antiviral drugs. This is called “antiviral resistance” and when that happens, the antiviral drug in question will be less effective in treating or preventing illnesses caused by that virus.
Influenza A viruses, including two subtypes (H1N1) and (H3N2), and influenza B viruses currently circulate worldwide, but the prevalence of each can vary among communities and within a single community over the course of an influenza season. Increases or changes in the patterns of antiviral resistance to the different types and subtypes of influenza viruses can mean that health care providers need to change their practices for prescribing antiviral medications.
CDC monitors circulating influenza strains for antiviral resistance year-round so it can promptly update public health recommendations when resistance develops. Oftentimes, antiviral resistance can develop very quickly. During the 2005-06 season, CDC found that a high percentage of influenza A (H3N2) viruses (92%) were resistant to amantadine and rimantadine. This trend has continued through the 2006-07, 2007-08 and 2008-09 seasons.
Resistance to the antiviral drug oseltamivir was first detected at very low levels during the 2006-07 flu season, when 0.7% of influenza A (H1N1) viruses tested by CDC were found to be resistant to this drug. This percentage increased to 10.9% during the 2007-08 season.
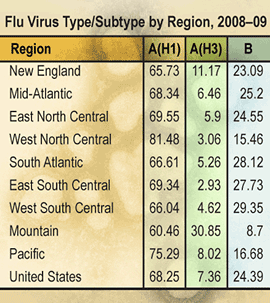
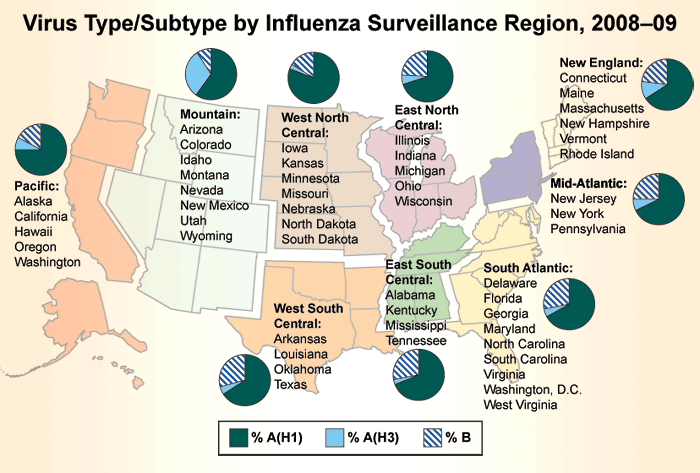
So far this season, approximately 68% of circulating influenza viruses reported to CDC through nationwide surveillance efforts have been influenza A (H1N1) viruses, approximately 7% have been A (H3N2) viruses and approximately 24% have been influenza B viruses. Since week 2 (the week ending January 17, 2009), when influenza activity began to increase nationally, influenza A (H1N1) viruses have predominated circulation nationally each week and for the season overall in all regions. Since week 2, 91% of subtyped influenza A viruses reported to CDC were influenza A (H1).
Since October 1, 2008, 364 influenza A (H1N1) viruses, 56 influenza A (H3N2) viruses, and 166 influenza B viruses have been tested for resistance to the neuraminidase inhibitor class of antiviral drugs, which includes oseltamivir (sold as Tamiflu®) and another drug, zanamivir (sold as Relenza®). In addition, 365 influenza A (H1N1) viruses and 56 influenza A (H3N2) viruses have been tested for resistance to the adamantanes (amantadine and rimantadine). The results of antiviral resistance testing performed on these viruses are summarized in the table below.
Isolates tested (n) |
Resistant Viruses, Number (%) |
Isolates tested (n) |
Resistant Viruses, Number (%) |
||
|---|---|---|---|---|---|
Oseltamivir |
Zanamivir |
Adamantanes |
|||
| Influenza A (H1N1) | 364 |
359 (98.6%) |
0 (0) |
365 |
3 (0.8%) |
| Influenza A (H3N2) | 56 | 0 (0) | 0 (0) |
56 |
56 (100%) |
| Influenza B | 166 | 0 (0) | 0 (0) |
N/A* |
N/A* |
*The adamantanes (amantadine and rimantadine) are not effective against influenza B viruses.
Influenza A (H1N1) viruses from 36 states have been tested for antiviral resistance to oseltamivir so far this season. To date, all influenza A (H3N2) viruses tested are resistant to the adamantanes and all oseltamivir-resistant influenza A (H1N1) viruses tested are sensitive to the adamantanes.
Resistance to the adamantanes (amantadine and rimantadine) remains low (0.8%) among circulating influenza A (H1N1) viruses.
Because of this increase in antiviral resistance to oseltamivir among circulating influenza A (H1N1) virus strains, CDC issued new Interim Antiviral Guidance for the 2008-09 season on December 19, 2008. For optimal protection against circulating influenza viruses, CDC recommends that providers be aware of influenza virus activity in their area, and consider treating patients with zanamivir, or alternatively, a combination of oseltamivir and rimantidine, when influenza A (H1N1) virus infection or exposure is suspected.

CDC asks that providers refer to the FluView Weekly U.S. Influenza Surveillance report throughout the season and contact their state public health department for information on local flu activity.


