|
Progress on Human Avian Flu Vaccine
 |
|
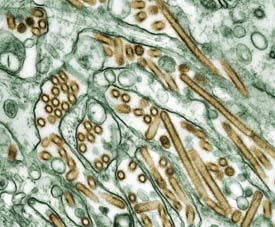
| Avian influenza viruses (gold) grown in culture cells (green). Image courtesy of Cynthia Goldsmith, Jacqueline Katz and Sherif R. Zaki, Centers for Disease Control and Prevention. |
|
When combined with an immune-boosting substance called an adjuvant, low doses of an experimental vaccine against a strain of avian influenza provoked a strong immune system response in humans. The result brings researchers one step closer to creating vaccines that can protect people from emerging avian flu viruses.
In 1999, two children in Hong Kong became infected with H9N2, a strain of avian influenza that hadn’t previously been detected in humans. People have little or no natural defenses against avian influenza viruses like H9N2 or the more deadly H5N1 because they’ve historically circulated only in birds. If one of these viruses were to acquire the ability to spread easily from person to person, it could cause a pandemic.
In 2004, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) asked Novartis Vaccines and Diagnostics (formerly Chiron Corporation) to produce an experimental H9N2 vaccine. Dr. Robert L. Atmar and his colleagues at the NIAID-supported Viral Respiratory Pathogens Research Unit at Baylor College of Medicine set out to test the vaccines in a Phase I clinical trial. Phase I vaccine trials are designed to assess a vaccine’s safety and ability to stimulate the immune system.
The researchers vaccinated 48 volunteers, aged 18 to 34, with H9N2 vaccine made from inactivated virus at one of four dosages. Another 48 people received vaccines at one of the same four dosages prepared with Novartis’s MF59 adjuvant, which is designed to boost the immune system’s reaction. The volunteers were vaccinated twice, with each shot 28 days apart. To measure the immune system responses, the researchers measured the levels of antibody each person produced against the virus. Antibodies are molecules produced by the immune system to help ward off infection. In general, the higher the level of antibodies made in response to a vaccine, the more protective the vaccine will be if the person later encounters the virus
Their results were published online on September 25, 2006, in Clinical Infectious Diseases. The side effects of the vaccinations were generally mild. Antibody levels in people who received the “adjuvanted” vaccine were significantly higher than in those who received the vaccine without adjuvant. The adjuvanted vaccine raised antibody levels after a single dose to ranges the researchers suggest may be able to protect people against infection. Phase I vaccine trials, however, aren’t designed to see whether a vaccine can prevent infection, so future studies will have to look at that question.
“The results of this clinical trial add to the growing body of information demonstrating the potential value of adjuvanted avian influenza vaccines,” says NIAID Director Dr. Anthony S. Fauci.
Currently, MF59 is licensed for use as a vaccine adjuvant in Europe but not in the U.S. These findings suggest that MF59 deserves further study.
|


