As mentioned earlier, one of the hallmarks of AD is the formation of NFTs, which consist largely of an abnormal form of tau. Long considered by many investigators to have a secondary role in AD, tau has, in recent years, come into its own as a leading player in AD research. Findings from the past several years clearly show why tau is generating new excitement.
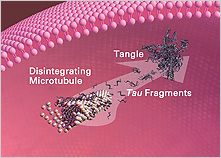 NFTs are found in a variety of human diseases other than AD, including corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17). These diseases are called “tauopathies.” Even though no mutations have been found in the tau gene in AD, inherited mutations do occur in other tauopathies that can change the structure of the protein from normal to abnormal. Previously, only transgenic mice that had been bred to have mutated tau demonstrated NFTs. Now, a research group at the Albert Einstein College of Medicine in New York, has developed a new mouse model of human AD (Andorfer et al., 2005). These “hTau” mice have non-mutant human tau protein and accumulate an excessive amount of an abnormal form of tau. They also form clumps of tau filaments in a region-specific fashion that is similar to AD. This new mouse model will allow researchers to investigate the relationship between cell death, accumulation of altered tau, and the development of NFTs. This study also provides compelling evidence that neuronal death in AD may not result directly or primarily from NFT formation, but rather from disrupted axon transport (in other words, a loss of normal tau function). The scientists found that the presence of tau filaments did not directly correlate with death within individual cells. Instead, they found that it was associated with the appearance of cell-cycle molecules and the initiation of DNA synthesis, which suggests that cell death can occur independently of NFT formation. Possibly, the neurodegeneration occurring in the hTau mice may be at least partially due to abnormal, incomplete initiation of the cell-cycle process (see the following section for more on the cell-cycle process).
NFTs are found in a variety of human diseases other than AD, including corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17). These diseases are called “tauopathies.” Even though no mutations have been found in the tau gene in AD, inherited mutations do occur in other tauopathies that can change the structure of the protein from normal to abnormal. Previously, only transgenic mice that had been bred to have mutated tau demonstrated NFTs. Now, a research group at the Albert Einstein College of Medicine in New York, has developed a new mouse model of human AD (Andorfer et al., 2005). These “hTau” mice have non-mutant human tau protein and accumulate an excessive amount of an abnormal form of tau. They also form clumps of tau filaments in a region-specific fashion that is similar to AD. This new mouse model will allow researchers to investigate the relationship between cell death, accumulation of altered tau, and the development of NFTs. This study also provides compelling evidence that neuronal death in AD may not result directly or primarily from NFT formation, but rather from disrupted axon transport (in other words, a loss of normal tau function). The scientists found that the presence of tau filaments did not directly correlate with death within individual cells. Instead, they found that it was associated with the appearance of cell-cycle molecules and the initiation of DNA synthesis, which suggests that cell death can occur independently of NFT formation. Possibly, the neurodegeneration occurring in the hTau mice may be at least partially due to abnormal, incomplete initiation of the cell-cycle process (see the following section for more on the cell-cycle process).
In a second tau advance, scientists at the University of Minnesota Medical School were able to partially rescue memory function in a transgenic mouse model that had a form of a mutant tau gene whose synthesis could be suppressed by a drug (SantaCruz et al., 2005). This meant that production of the mutant tau could be precisely regulated. As the mice aged, they produced more mutant tau and began to accumulate NFTs. Their neurons began to die, brain tissue shrank, and memory was lost. The researchers were able to stop the production of the mutant tau by giving the mice the drug that turned off the gene. Once the mutant gene was suppressed, the scientists found, much to their surprise, that not only did the memory loss stop, it actually was partially reversed. Even more striking, memory function improved even though NFTs formed from tau that had already been made continued to accumulate in the brains of the mice. The fact that memory function improved in mice carrying the mutant tau gene when the gene was turned off, despite continued NFT accumulation, implies that the processes that lead to memory loss and those that cause NFTs are separate. Perhaps NFTs do not invariably cause neuronal death, but an earlier, toxic form of abnormal tau does. Some investigators are suggesting that NFTs, like beta-amyloid plaques, may even be a protective response by the brain that is aimed at preventing abnormal tau from damaging the neuron (Tanzi, 2005).
Tau studies are one of the most active areas of AD research, and, as with other areas of AD research, new findings are emerging all the time. Current and future studies in animal models are examining whether it might, in fact, be possible to “turn on and off’ the synthesis of abnormal, damaging tau and beta-amyloid and exploring whether the brain could even regain some cognitive function once the disease process has begun.
New Evidence Links Beta-Amyloid and Tau
Immunizing against the beta-amyloid peptide is a promising therapeutic approach (see "The Emerging Field of AD Translational Research" for a description of current research in this area). Several studies have demonstrated in mouse models of AD that this approach, called “beta-amyloid immunotherapy,” leads to a dramatic reduction in beta-amyloid deposition in the brain. The availability of new brain imaging techniques and mouse models that have both beta-amyloid and tau pathology has made it possible to tackle some key questions about the pathologic processes that may be alleviated by beta-amyloid immunotherapy and about how damage from beta-amyloid and tau relate to one another.
Scientists from the University of California at Irvine injected anti-beta-amyloid antibodies in the brains of transgenic mice that develop both beta-amyloid deposits and NFTs. This treatment led to a rapid reduction of beta-amyloid deposits outside as well as inside neurons and reversed the early signs of tau pathology, namely accumulation of abnormal tau within neuronal bodies and dendrites (Oddo et al., 2004). When the anti-beta-amyloid antibodies were removed, the beta-amyloid pathology re-emerged. This was followed by the reappearance of tau pathology. In a follow-up study, these investigators used anti-beta-amyloid antibodies to determine which aspect of beta-amyloid pathology coincides with early cognitive impairments in the same animal model. They found that clearance of beta-amyloid accumulations within the neurons was correlated with reverses in the early signs of cognitive dysfunction (Billings et al. 2005).
These findings from animal models provide proof that beta-amyloid and tau pathology are linked in that levels of beta-amyloid deposits influence levels of NFTs. These findings also show that the accumulation of beta-amyloid within the neuron occurs before any apparent beta-amyloid deposits and coincides with early signs of cognitive impairment. |
<< Back | Next >>