Justification
Authorizing Legislation: Section 301 of the Public Health Service Act, as amended. Reauthorizing legislation will be submitted.
Budget Authority
| |
|---|
| Current Law | $786,056,000 | $893,443,000 | $893,130,000 | $968,699,000 | $75,745,000 |
| BA |
| Accrued Costs | 2,711,000 | 2,934,000 | 2,934,000 | 3,010,000 | 76,000 |
| Proposed Law | 788,767,000 | 896,377,000 | 896,064,000 | 971,709,000 | 75,569,000 |
| BA |
| FTE | 395 | 445 | 445 | 444 | (1) |
Introduction
This document provides justification for the FY 2003 activities of the National Institute on Aging (NIA), including HIV/AIDS-related activities. A detailed description of the NIH-wide FY 2003 HIV/AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)."
The President's appropriations request of $971,709,000 for this account includes current law adjusted by assuming Congressional action on the proposed Managerial Flexibility Act of 2001.
At 77, former U. S. President Jimmy Carter writes, lectures, travels extensively, and spearheads numerous humanitarian activities. Award-winning author Madeleine L'Engle published two new books this year; she's 83. 89-year-old Julia Child continues to practice and promote the gourmet cookery that made her a household name. And legendary runner Johnny Kelley, now 94, gave up running marathons about 10 years ago, but continues to participate in shorter races and serves each year as the grand marshal of the Boston Marathon.
These are only a few of the millions of Americans over age 65 who remain healthy, active, and productive well into old age. More people than ever before are enjoying robust health and productivity well into their seventies, eighties, and even beyond. Life expectancy, around 49 years in 1900, has increased over the past century to about 76, thanks to improvements in health care, nutrition, and the overall standard of living for most people, and nearly three quarters of people 65 and older rate their health as "good," "very good," or "excellent."
But good health is far from a universal reality for older Americans. The latest national surveys indicate that about one-fifth of people age 65 and older—more than 7 million people—report some disability. Chronic disease, memory impairment, and depressive symptoms affect large numbers of older people and the risk of such problems significantly rises with age. Nearly half of those age 85 and older suffer from Alzheimer's disease. These millions of less fortunate older people struggle with daily activities as simple as bathing and dressing, with families and friends taking on the difficult and often costly role of caregiver.
Understanding the differences between advanced years that are active and independent and those that are characterized by frailty and dependence is at the heart of the NIA's research program. Since the Institute's founding in 1974, research has shed considerable light on aging and health. It is now known that aging itself is not the cause of disease, disability, and frailty. Indeed, the converse is true: It is disease and disabling processes, influenced by age-related changes in the body and by unhealthy choices and sedentary lifestyles, that are the most important factors in compromising the quality of life for older people. This fundamental shift in thinking was reinforced most recently with insights from the National Long Term Care Survey (NLTCS). According to this study, the rate of disability among older Americans dramatically declined from the 1980s through the mid 1990s, even among people age 85 and older. These findings, along with evidence from a number of clinical trials and studies testing specific interventions, suggest more strongly than ever that disease and disability are not inevitable consequences of aging.
The challenge now is to maintain and even accelerate the trend in declining disability and to reduce rates of disease amid a steep rise in the number and proportion of older people. The task is urgent. Demographic projections show that the U.S. population is beginning to age at a rapid pace, with the first baby boomers turning 65 in 2011. Between now and the year 2030, the number of individuals age 65 and older likely will double, reaching 70.3 million and comprising a larger proportion of the entire population, up from 13 percent today to 20 percent in 2030. Of great interest is the explosive growth anticipated among those most at risk of disease and disability, people age 85 and older. Their ranks are expected to grow from 4.3 million in 2000 to at least 19.4 million in 2050. The racial and ethnic makeup of the older population will change dramatically as well, creating a more diverse population of older Americans. These demographic factors combined threaten to increase the burden of age-related diseases and conditions on individuals, families, and society. Unless new understandings and interventions are developed and implemented to reduce disease and disability, the costs, in both human and financial terms, could be extraordinary.
In the 20th century, health research and public health practices did much to extend life and improve health. At the start of this new millennium, the NIA's research portfolio is aimed primarily at increasing "healthspan," or years of healthy active life expectancy. Aging research is well poised to build upon the work of recent years to improve the lives of older Americans and their families. Toward that end, NIA's overall program is wide-ranging and includes research on: the biochemical, genetic, and physiological mechanisms of aging in humans and animal models; the structure and function of the aging nervous system; social and behavioral aspects of aging processes and the place of older people in society; and the pathophysiology, diagnosis, treatment, and prevention of age-related diseases, degenerative conditions, and disabilities. The NIA is also the lead federal agency for Alzheimer's disease research. In close collaboration with the National Advisory Council on Aging and other public and private organizations, the NIA has developed a strategic plan for aging research, to identify goals for the years 2001–2005. These goals address scientific areas with the greatest promise for advancing knowledge, many outlined in this narrative. The NIA also recently completed a strategic plan on disparities in health status of older Americans of different racial and ethnic backgrounds. In this narrative, the Institute focuses on recent progress and future directions for research in four key areas: Section I) Alzheimer's disease and the neuroscience of aging; Section II) reducing disease and disability; Section III) the biology of aging; and Section IV) the behavioral and social aspects of growing older. In all of its efforts, the Institute is paying special attention to reducing health disparities among different groups of Americans (Section V).
Alzheimer's Disease and the Neuroscience of Aging
Alzheimer's disease (AD) is a progressive, currently irreversible brain disorder. People with AD gradually suffer memory loss and a decline in thinking abilities, as well as major personality changes. These losses in cognitive function are accompanied by pathologic changes in the brain, including the buildup of insoluble protein deposits called amyloid plaques and the development of neurofibrillary tangles, which are abnormal collections of twisted protein threads found inside nerve cells. Such changes result in death of brain cells and breakdown of the connections between them. AD advances gradually but inexorably, from early, mild forgetfulness to a severe loss of mental function called dementia. Eventually, people with AD become dependent on others for every aspect of their care. The risk of developing AD increases exponentially with age, but it is not a part of normal aging.
AD is the most common cause of dementia among people age 65 and older and is a major public health issue for the United States because of its enormous impact on individuals, families, the health care system, and society as a whole. Scientists estimate that as many as 4 million people currently suffer with the disease, and annual costs associated with AD are estimated to exceed $100 billion. 5 6 As the population ages, the numbers of people with AD and costs associated with increased prevalence could rise significantly.
The following section on Alzheimer's disease and related neuroscience describes recent advances in seven areas of AD research: early diagnosis, normal age-related cognitive change, the role of environmental factors in the development of AD, new animal models that may provide insights into the etiology of AD, preclinical studies of new preventive and therapeutic agents, clinical trials to test new therapies that may delay or prevent development of the disease, and studies related to easing caregivers' burdens.
Early Diagnosis of AD
Early diagnosis of AD benefits affected individuals and their families, clinicians, and researchers. For patients and their families, a definitive early diagnosis provides an opportunity to plan and to pursue options for treatment and care while the patient can still take an active role in decisionmaking. For clinicians, accurate early diagnosis facilitates the selection of appropriate treatments, particularly as new interventions are developed to stop or slow progression of symptoms. And for researchers, earlier and more accurate diagnosis may simplify clinical studies of new therapies and preventive measures by allowing early intervention, when cognitive loss is less severe and, consequently, the response is more easily measured. Research suggests that the earliest AD pathology may begin to develop in the brain 10 to 20 years before clinical symptoms yield a diagnosis. Scientists have made tremendous progress looking for ways to diagnose AD in its pre-symptomatic or pre-clinical stages, including reliable, valid, and easily attainable biological markers that can identify cases very early in the course of disease when treatment may be more effective. Eventually, combinations of specific strategies to image the brain, along with genetic, clinical, and neuropsychological assessments may become the key to identifying people at very high risk of developing AD.
Identification of Risk Factor Genes for Late-Onset Alzheimer's Disease. Until 2001, just four of the approximately 30,000 genes in the human genome were conclusively known to affect the development of AD pathology. Three of these genes are associated with early onset AD, and only one is associated with the more common form of the disease, late-onset AD. Recent genetic studies suggest that as many as four additional and as yet unidentified genes may also be risk factors for late-onset AD. Finding new risk factor genes will help identify pathways affecting the development or progression of AD and may eventually lead to better predictors of the disease even before it is clinically apparent.
Imaging Small Regions of the Brain in Humans and Genetically Modified Mice. Functional imaging, or the visualization of processes within the body in "real time," is potentially a useful tool for detecting changes in the brain that may suggest early AD, or for identifying markers that may indicate the extent of the disease. However, barriers to its optimal use remain. Traditional functional magnetic resonance imaging (fMRI), a common method for visualizing brain structures, allows imaging of structures a few millimeters in size, but no smaller. This resolution is insufficient for evaluating smaller structures, such as subregions of the hippocampus that are important to learning and memory. In addition, it requires that the person being imaged respond to specific instructions, an impossibility for many with cognitive impairments. A "new" method of fMRI has been developed that permits evaluation of these minute areas of the brain. In addition, this method, which is dependent on oxygen use in the brain during rest, does not require the person being studied to perform a mental task, making it easier to use among cognitively-impaired people. Although fMRI is currently available only in a research setting, these techniques could eventually be used to identify persons with loss of neurons in very specific brain regions—for example, in identification of persons at risk for developing AD.
Loss of Neurons in a Particular Brain Region is Associated with Onset of Cognitive Decline in Older Individuals. Participants in a recent study had detailed clinical evaluations within 12 months of death and were categorized as having no cognitive impairment, mild cognitive impairment (MCI—often a precursor of AD), or mild to moderate AD. At autopsy, people with MCI all had significant losses of neurons in the entorhinal cortex (EC) of the brain relative to those with no cognitive impairment, and these losses were as extensive as those in the patients with full-blown AD. In a second study, autopsies were done on people whose cognitive status had been assessed shortly before their death. This study found extensive loss of neurons in the EC in people with very mild AD. In contrast, those with no loss of neurons in the EC, but with plaques and tangles in their brains (the hallmarks of AD), showed no cognitive decline. These findings indicate that elderly people with MCI and very mild AD already have dramatic decreases in the number of neurons in a particular region of the EC, and that it is the onset of neuronal loss, rather than the development of plaques and tangles alone, that is associated with the onset of AD-related memory loss. This suggests that the development of interventions that will prevent, delay, or slow the degeneration of these critical neurons may be extremely beneficial to people at risk of AD.
Normal Age-Related Cognitive Change
While most people remain alert and mentally able as they age, some age-related changes in memory, learning, and attention are normal. Improved characterization of normal cognitive function and underlying brain changes throughout life will help us distinguish normal from abnormal age-related cognitive changes. A better understanding of what is "normal" and what is not may aid the early diagnosis of AD; it could also alleviate the anxiety of people who observe modest but perceptible changes in cognitive function in themselves or a loved one and fear that such changes are the harbingers of a decline into dementia.
Some People with Mild Cognitive Impairment (MCI) Progress to Alzheimer's Disease and Some Don't: How To Tell. Researchers evaluated people with MCI and, based on clinical findings, categorized their MCI as representing probable AD-related dementia, "incipient" AD-related dementia, or "uncertain" AD-related dementia. These volunteers were reassessed annually for up to 9.5 years, and at that time all the volunteers whose clinical findings had indicated probable AD-related dementia had developed the clinical symptoms of AD. However, many in the less severe groups had not. In another study, investigators categorized people as having normal cognition or having MCI or probable AD, both at entry into the study and at a subsequent clinical evaluation 2-4 years later. Each participant had an MRI scan at baseline and at follow up. The size of the hippocampus decreased in all groups, most rapidly in AD patients, less rapidly in those with MCI, and least in the control group. Within the control and MCI groups, those who experienced decline in cognitive function over time had a significantly greater decrease in hippocampal size than those who remained clinically stable. Previous studies have shown that baseline hippocampal volume can provide predictive information about which patients with MCI will decline to AD versus which will remain stable; together with this baseline information, serial measurements of hippocampal size through non-invasive MRIs may be a useful tool in the future for identifying people with MCI who will and won't progress to AD.
Early Life/Environmental Factors and AD
Environment May Protect Against Cognitive Decline and Alzheimer's Disease. Investigators hypothesized that recreational activities would be an excellent measure of mental activity, as these are less strongly influenced by economic and social factors than other risk factors for AD, such as the number of years of formal education. They recorded the extent to which 500 AD patients and age-matched healthy people had participated in recreational activities over their adult life. Patients with AD were found to have been much less active than healthy people of similar background in terms of both diversity and intensity of recreational activities engaged in during early and middle adulthood. These differences were not related to differing educational or income levels, age, or gender. People who were relatively inactive in midlife had a two and a half fold increased risk of developing AD. In a separate study, the relationship of social ties and support to patterns of cognitive aging over a 7.5 year period was examined in 1200 high functioning, community-dwelling adults aged 70-79. The results showed that greater baseline emotional support was a significant predictor of better-maintained cognitive function at the 7.5-year follow-up, controlling for known socio-demographic, behavioral, psychological, and health status predictors of cognitive aging.
Animal Models of Neurodegenerative Disease
Animal models that mimic human disease are central to research for many reasons. Animals and humans share many genetic and physiologic features, so experimental results obtained in animals can frequently (although not always) be extrapolated to humans. It is much easier to create specific genetic mutations and observe their effects in animals than to search for them in humans, and because the lifespan of most animals is relatively short, it is easier to observe the effects of those mutations over several generations. Recently, scientists have created several new models for research on neurodegenerative diseases, including AD.
The TAPP Mouse: The First Link Between Plaque and Tangle Formation. The neurofibrillary tangles (NFTs) that characterize AD are composed primarily of a form of the protein called tau. In addition to NFTs, another key feature of AD is the deposition of beta-amyloid (Aß) in insoluble amyloid plaques outside brain cells. The fragment is formed by clipping it out of the much larger amyloid precursor protein (APP). Although many scientists believe that excess production of Aß is a root cause of AD, it is still unclear how this causes pathology. In particular, scientists do not understand whether excess production of Aß leads to development of NFTs.
Now, a new mouse model may help us answer this question. A number of transgenic mouse models of AD have been developed by inserting human mutated APP genes into mice. Amyloid plaques, but not NFTs, form in these mice. A model for pathology of the tau gene has also been developed, but the NFTs in these mice do not usually form in areas of the brain that are vulnerable to AD. Scientists recently crossbred the tau mutant mice with the APP mutant mice to produce a new model, the TAPP mouse. The TAPP mice produce amyloid plaques; they also produce NFTs in regions of the brain that are vulnerable to AD, suggesting that APP or Aß peptide can influence the regional formation of NFTs. This is the first animal model in which the elusive connection between amyloid pathology and tangle formation can be investigated.
This improved animal model for AD may also be critical for success in developing therapies against NFT formation and the death of neurons in AD brain.
Pesticide Creates a Rat Model of Parkinson's Disease. Parkinson's disease is a progressive neurodegenerative disorder characterized by selective death of neurons that make the neurotransmitter dopamine in a region of the brain called the substantia nigra. In an effort to develop a new model of Parkinson's disease, scientists exposed rats to rotenone, a common pesticide. Exposed rats showed pathological changes characteristic of Parkinson's disease, as well as motor behavior abnormalities, such as rigidity and decreased motor activity, that are frequently seen in Parkinson's disease patients. This new model of Parkinson's disease will be useful in designing and testing new therapeutic interventions, as well as further identifying environmental exposures that may be risk factors for developing the disease.
Preclinical Research
There are currently no effective, generally useful treatments for Alzheimer's disease; i.e., a treatment that works on large numbers of patients, that maintains its effectiveness for a long period, that works in both early and late stages of the disease, that improves functioning of patients in activities of daily living as well as on sensitive neuropsychological measurements, and that has no serious side effects. In addition, none of the treatments presently approved for AD alter the progressive underlying pathology of the disease. One way to treat the disease successfully may be to interfere with early pathological changes in the brain, including the development of amyloid deposits and the formation of neurofibrillary tangles. A number of promising approaches, many of them targeted at the reduction of amyloid plaques, are currently being developed and tested in various model systems. If these approaches prove safe and effective in animals, studies in humans could follow.
Promising Immune Treatment for AD. Using mice that were genetically engineered to produce Aß and develop AD-like pathology in the brain, investigators found that treatment with an antibody that recognizes Aß peptide results in clearance of Aß plaques from the brain. Based on these findings, the investigators hypothesize that treatment with antibodies may be a useful and important approach for the treatment and prevention of AD and other neurodegenerative diseases.
Phenserine Regulates Translation of $-Amyloid Peptide Message: A New Target for Alzheimer's Disease Drug Development. AD's tell-tale amyloid $-peptide (Ab) plaques are formed when a larger protein called amyloid precursor protein (APP) breaks down. Researchers are working to develop agents that reduce APP expression. In a recent study, investigators conducted laboratory tests of a drug called phenserine, originally developed to increase levels of the chemical messenger acetylcholine, which is depleted in the brains of people with AD. They discovered that phenserine inhibits APP formation in cells through a mechanism independent of acetylcholine. Current research is directed towards the design, synthesis, and development of agents that optimally and safely regulate APP and Aß levels with the aim of slowing or halting the molecular events that lead to AD.
Statins May Reduce the Risk of AD. Evidence increasingly suggests that high levels of cholesterol may have a role in the development of AD. Two recent studies found that the use of statins, the most common type of cholesterol-lowering drugs, may lower the risk of developing AD. In a third study, transgenic mice fed a high cholesterol diet had much higher levels of blood cholesterol and the mean number of amyloid deposits in their brains (a hallmark of AD) was 65 percent higher than those on the normal diet. Taken together, these results suggest that statins—or dietary interventions—may be effective treatments or preventives for AD.
Imaging Clearance of Plaques by Immunotherapy in Living Mice. Researchers have developed a powerful new imaging technique, multiphoton microscopy, that enables them to view changes in the brain caused by AD and subsequent changes induced by treatment. Multiphoton microscopy provides a resolving power 100 times greater than that of other noninvasive imaging techniques, and allows sufficient resolution to view very small structures and lesions in the brain such as plaques. In a recent experiment, antibodies specific for amyloid and labeled with a fluorescent dye were placed directly onto the surface of brains of anesthetized mice who had developed AD-like plaques. Using the new imaging technique, scientists noted reversal of existing amyloid-$ deposits in the brain within 3 days of treatment with the antibodies. These findings demonstrate the potential effectiveness of antibody-mediated passive immunization for the removal of plaques from the brain.
BACE1 is the Major Beta-Secretase for Generation of Amyloid-beta Peptides in Mouse Brain. A major focus of study has been the process by which the amyloid precursor protein (APP) is clipped apart by enzymes to release Ab fragments, which are then deposited in the brain as AD's characteristic plaques. A recently discovered enzyme that helps clip Ab out of the APP protein was given the name b-secretase. In order to identify the enzyme that is responsible for production of Aß in the brain, scientists developed a mouse model in which the gene for the BACE1 enzyme, a candidate for the active b-secretase, was selectively eliminated to see whether removing it would interfere with the clipping of APP to produce amyloid. Indeed, Aß peptides were no longer produced in brain cell cultures made from the "knockout" mice, suggesting that BACE1 is responsible for the cleavage of APP into Aß in the mouse brain. These findings will help in design of drugs to inhibit b-secretase activity, in hope of slowing plaque production. Furthermore, because the mice in which the BACE1 gene has been eliminated seem to develop normally, it may be possible to develop BACE1 inhibitors that interfere with Aß deposition without negative effects on other metabolic pathways in brain or other tissues.
Clinical Trials
Today, the few FDA-approved drug treatments for AD maintain cognitive function in AD patients in only a subset of patients and for only a limited time. However, an estimated 50 to 60 compounds are presently or will soon be tested in human AD clinical trials. These studies are sponsored by a number of sources, including the NIA, other NIH institutes, and the private sector, primarily pharmaceutical companies. Compounds now under scrutiny focus on three major areas of treatment: short-term maintenance of cognitive function; slowing the progress of the disease, delaying AD's onset, or preventing the disease altogether; and managing behavioral problems associated with AD.
Interest is currently focusing on compounds that directly target disease-related pathologies. A rapidly evolving research focus lies in prevention trials, and a number are underway to test the effectiveness of therapies in people without symptoms or who have only slight memory problems. Recruitment is now complete for the first NIH AD prevention trial, which will take place at more than 70 sites across the U. S. This trial compares the effects of vitamin E and donepezil (brand name Aricept) in preventing the development of AD in people diagnosed with mild cognitive impairment, a population at high risk for developing AD. Further examination of estrogen and studies of various classes of anti-inflammatory drugs and antioxidants are also ongoing, and as scientists test these currently available medications, the next generation of drugs is being developed, targeting specific abnormal cellular pathways uncovered by recent discoveries, including plaque and tangle formation and death of brain cells. Prevention trials are among the most costly of research projects, but, if successful, the payoff in terms of reduced disease and disability will be significant.
Caregiving of AD Patients
Most of the approximately 4 million Americans with Alzheimer's today are cared for outside the institutional setting by an adult child or in-law, a spouse, another relative, or a friend. Caregivers frequently experience significant emotional stress, physical strain, and financial burdens, yet they often do not receive adequate support. Several recent studies have explored the problems faced by caregivers of AD patients, as well as possible interventions to reduce their burdens.
Depression and Agitation in Alzheimer's Disease: Effects on Caregivers. Previous research has examined the factors contributing to stress and depression in caregivers of an AD family member. A recent study found that the greater the level of depression in the patient, the greater the level of depression in the caregiver. Wives of AD patients and caregivers in poor health themselves were at particular risk for depression. This study demonstrates that the well-being of the caregiver and care recipient are closely related. The findings also support interventions for caregivers early in the family member's illness.
Women Caring for a Family Member with Dementia Can Benefit from an Exercise or Nutrition Program. A one-year study involved 100 women age 49 to 82 years who were sedentary, free of cardiovascular disease, and caring for a relative with dementia. Participants received either a home-based, telephone-supervised moderate-intensity exercise training or nutrition education program. Exercise consisted of brisk walking for four 30-to 40-minute sessions per week. Compared with the nutrition education group, caregivers who exercised showed significant improvements in physical activity levels, stress-induced blood pressure reactions, and sleep quality. The nutrition group reported significant reductions in percentages of total calories from fats compared to exercisers. Both groups reported significant improvements in psychological distress, including depressive symptoms and self-rated stress level.
Selected Future Research Directions in AD and the Neuroscience of Aging
National Cell Repository Expansion. To facilitate the identification of AD risk factor genes, NIA is planning the expansion of its National Cell Repository. A national resource for research on AD, the Repository was created to collect DNA, cells, and information from families with multiple affected individuals with AD. Its activities include the production of a catalog of cell lines and DNA samples that are available for qualified scientists to study. Because most researchers rely on extended families, sibling pairs, or case-control studies to look for genes, the goal of the expanded Repository will be to develop cell lines and collect data from persons identified through the Alzheimer's Centers who fall within one of the three categories. While previous research has emphasized the identification of genes associated with the familial form of the disease, this expansion will allow us to more rapidly identify the underlying genetic mechanisms of the more common late-onset form of AD.
Alzheimer's Disease Cooperative Study. NIA's major AD clinical trials effort is the Alzheimer's Disease Cooperative Study (ADCS), a consortium of 83 medical research centers in the U. S. and Canada. Established in 1991, the ADCS conducts clinical trials on compounds in which large pharmaceutical companies are generally not interested, including drugs that were patented and marketed for another use but might be useful for treatment of AD, or novel compounds from investigators with inadequate resources for clinical trials. During its first decade, the ADCS established a research infrastructure to carry out clinical trials for promising new therapies for AD, developed new and more reliable ways to evaluate patients enrolled in these and other studies, and initiated a number of clinical trials. The latest 5-year award will allow that work to continue and will move AD treatment research in new directions, including the study of a cholesterol-lowering statin drug, an antioxidant, and a high-dose vitamin regimen. Studies of interventions to combat behavioral and other manifestations of AD, including agitation and sleep disturbances, are also ongoing. In addition, the ADCS will develop and refine evaluation tools and methodologies for AD prevention research.
Extending Caregiving Research to Clinical Practice. NIA will expand the research scope and objectives of its Resources for Enhancing Alzheimer's Caregiver Health (REACH) program to extend promising caregiver interventions to the clinical setting. The REACH program is a large-scale, coordinated study to examine the effectiveness of social, behavioral, environmental and technological interventions for reducing caregiving burdens of caring for persons with dementia. In the first phase of the program (1995-2001), investigators at six sites tested a series of interventions. Now, in Phase II, the outcomes of these exploratory interventions will be analyzed and used to develop intervention strategies for further evaluation across multiple sites. Of particular importance is the program's emphasis on evaluating these interventions in diverse racial and ethnic populations.
Reducing Disease and Disability
Apart from AD, chronic disease and disability can compromise the quality of life for older people. Some 79 percent of people age 70 and older have at least one of seven potentially disabling chronic conditions (arthritis, hypertension, heart disease, diabetes, respiratory diseases, stroke, and cancer). The burden of such chronic conditions poses a challenge to individuals as well as families, employers, and the health care system. Research to improve understanding of the risk and protective factors for chronic disease and disability can offer effective prevention strategies. This section describes some of the latest findings on the treatment and prevention of various age-related diseases, as well as the molecular underpinnings of disease.
Treatment and Prevention of Disease
Treatment of disease in older people can be complicated by the presence of other diseases and disorders and by the use of multiple medications to treat various conditions. Potential interactions of medications, including those of prescribed drugs with over-the-counter drugs and dietary supplements, represent additional concerns. Moreover, adherence to treatment regimens can be difficult, as older patients often must maintain a complex schedule for taking several different medications. Research is ongoing to determine the best treatment approaches for older patients, particularly those with concurrent medical conditions, and to identify strategies for improving adherence and minimizing potentially adverse effects of medications.
Comorbidity and Breast Cancer in Older Women. Most breast cancer and related deaths occur in women aged 55 years and older. Concurrent age-related health problems such as hypertension, heart disease, diabetes, chronic obstructive pulmonary disease, and cerebrovascular disease are likely to affect the course of the disease and treatment options. Researchers have found that older breast cancer patients with preexisting health conditions receive less aggressive pretreatment assessments and cancer treatment than younger, healthier women. Given the high incidence and mortality rates of breast cancer in older women, research is needed to determine how age differences and accompanying health problems should guide assessment and treatment choices.
Low-Dose Estrogen Reduces Bone Breakdown in Older Women. More than 100 women over the age of 65 participated in a study of three different doses of estrogen (17b estradiol) therapy. The highest of these doses was the amount most commonly used today in estrogen replacement therapy, and the lowest dose was one-fourth this amount. The participants were studied for 6 months: 3 months on treatment and 3 months off. The low dose markedly reduced bone breakdown as measured by several serum markers, a reduction that was similar to that produced by the highest estrogen dose. Breast tenderness, bleeding, and thickening of the lining of the uterus (an indicator of potential adverse uterine effects), were significantly less frequent with the lowest dose. In fact, low-dose therapy resulted in no more side effects than placebo. These findings suggest that a lower dose of estrogen may be just as effective as the regular dose, but have fewer side effects.
Persistence of Cognitive Decline after Coronary Artery Bypass Surgery. One of the more common types of surgeries performed in the elderly is coronary artery bypass grafting (CABG). CABG may have adverse effects on the brain including stroke, post-operative delirium, and short-term cognitive impairment. Until recently, it was believed that most cognitive decline after CABG surgery is transient. However, researchers have now found that among older individuals undergoing CABG surgery, cognitive function at discharge may predict long-term cognitive function. Following a group of 261 CABG patients, the researchers found that over half exhibited some cognitive decline at discharge. The patients, as a group, went on to show a pattern of early improvement at 6 weeks and 6 months. At the 5-year assessment, however, some 42 percent of the surgery group was performing below baseline cognitive levels. Additional predictors of later decline included older age at surgery and lower level of education. Perioperative injury, increased susceptibility to injury, or decreased ability to recover from injury, may be responsible for cognitive dysfunction after CABG surgery and will be important research issues to pursue.
Physical Exercise Prevents Disability in Older People with Arthritis. Older people with osteoarthritis of the knee often have difficulty doing basic activities of daily living (ADLs), including walking, eating, dressing, using the toilet, bathing, or even moving from bed to a chair. Although previous exercise interventions have shown positive effects, none has yet been shown to affect clinically significant outcomes such as ADLs. Researchers recently conducted a study of exercise in 250 community-dwelling people, 60 years of age or older, with knee osteoarthritis. The participants were divided into 3 groups. Two groups participated in either an aerobic exercise program to increase endurance, or a resistance training program to increase strength. The third group did not participate in structured exercise programs and served as a control group. ADL disability was measured every 3 months throughout the 18 months of the study. Participants in both exercise programs had lower incidences of ADL disability than those in the control group. Individuals who complied most diligently with the exercise program had the lowest risk for disability. These results suggest that regular exercise has great potential to prolong the independence of older people despite the presence of this common and often disabling disease.
Reducing Delirium after Hip Fracture in Older Adults. Delirium, an acute confusional state, complicates recovery from hip fracture repair in at least one-third of the 250,000 older Americans who fracture a hip each year. Besides being frightening to patients and their families, and difficult to manage in the hospital, delirium after hip fracture is also associated with poor recovery of function. In a recent study aimed at reducing risk factors for delirium, geriatricians provided a variety of recommendations to the orthopedic physicians caring for the hip-fracture patients. These recommendations included transfusing blood to maintain an adequate red blood cell count, limiting the use of psychoactive medications, and providing adequate pain management. This intervention led to a one-third reduction in patients who developed delirium and a one-half reduction in the proportion who developed severe delirium compared to a control group of patients. This study demonstrates that measures can be taken to prevent delirium in vulnerable older patients.
Dietary Restriction Increases Levels of Growth Factors in the Brain and Stimulates Production of New Nerve Cells. Reducing calorie intake (dietary restriction, or DR) can increase the lifespan of rodents, and can also promote resistance of rodents' brain cells to injury. The cellular and molecular mechanisms responsible for the beneficial effects of DR on the brain are unknown. In a recent study, adult rats and mice maintained on a DR feeding regimen for 3 months showed increased levels of the neuronal growth factor brain-derived neurotrophic factor (BDNF) in the hippocampus, a brain region involved in learning and memory, as well as in several other brain regions. The rats also exhibited a significant increase in the numbers of newly divided cells in a region of the hippocampus. These findings provide the first evidence that diet can affect expression of a neurotrophic factor and can also stimulate the production of new neurons in the brain; they may also help to explain the beneficial effects of DR on learning and memory in animals, and may have implications for developing new ways to combat age-related neurodegenerative disorders.
Working to Cure Prion Diseases. Prions are infectious proteins that alter the shape of a normal cellular protein, changing it into a prion. They can cause several rare but invariably fatal neurodegenerative disorders, including Creutzfeld-Jakob disease (CJD). Investigators have used a number of approaches to identify compounds that are effective in clearing prions from cells in tissue culture. Two drugs, quinacrine (an anti-malarial drug) and chlorpromazine (an anti-psychotic drug), are known to enter the brain and are among the compounds that cause the clearance of prions in tissue culture. These compounds were effective at non-toxic concentrations and have been used for many years in humans, making them likely subjects for clinical trials to test their efficacy in treating people with CJD who otherwise face certain death.
Potential New Treatment for Type 2 Diabetes Mellitus in the Elderly. Type 2 diabetes mellitus is the most common form of diabetes among the elderly. It occurs when pancreatic beta cells produce insufficient insulin or when the body cannot use its insulin efficiently. GLP-1, a gut peptide, can stimulate beta cells to produce more insulin in middle-aged people with type 2 diabetes. However, until recently it had not been tested in older adults, who make up the majority of patients with the disease. Recent studies showed that GLP-1 potently stimulated insulin release in the elderly and lowered blood glucose to normal levels. In parallel studies with elderly rodents, investigators found that when GLP-1 was given long-term, it increased the number and activity of pancreatic beta cells. Researchers are now developing longer-acting forms of GLP-1 and have begun longer-term trials of GLP-1 treatment in an elderly population.
Molecular Understanding of Disease Processes
A Large-Scale Analysis of Gene Expression in Ovarian Cancer Suggests Possible Targets for Early Detection and Treatment. Ovarian cancer is the fifth most common cause of cancer death among women in the United States, yet it is very poorly understood. Ovarian cancer affects older women disproportionately. Because there are few early symptoms, and no sensitive screening tests for use in the general population, it is typically diagnosed in late stages, when treatment is difficult and often unsuccessful. More detailed knowledge of gene expression in ovarian cancer is crucial to a better understanding of how ovarian tumors form and to identifying novel targets for diagnosis and therapy. Serial Analysis of Gene Expression (SAGE) is a powerful method for analyzing the genes expressed in any cell or tissue. Researchers developed SAGE libraries representing the genes active in various normal and neoplastic ovarian tissues and identified many genes that were expressed differently in normal ovary and in ovarian cancer cells. The genes identified by this method have the potential to become useful targets for early detection and therapy. In addition, this work provides a framework for a detailed understanding of how ovarian tumors form at the molecular level.
Integration of Aging and Cancer Research. Cancer is largely a disease of the elderly. However, much remains unknown about cancer diagnosis, prevention, and treatment in older people. NIA and the National Cancer Institute (NCI) have created a partnership that has resulted in support for a number of projects. Ongoing initiatives include joint program announcements and the inclusion of NIA-supported studies within the NCI Cooperative Group system (a network of consortia throughout the U. S. that collaborate frequently on clinical trials for a variety of common cancers). Recently, the NIA and NCI extended this collaboration to NCI's Cancer Centers program, which is composed of 60 major academic and research institutions that sustain broad-based, coordinated, interdisciplinary programs in cancer research. A joint workshop was held in June 2001, and an aggressive research agenda within the NCI-designated cancer centers is now developing that can reduce the burden of cancer for older persons.
Biology of Aging
Aging is accompanied by gradual changes in most body systems. Research on the biology of aging focuses on understanding the cellular and molecular processes underlying these changes as well as those accompanying the onset of age-related diseases. As scientists learn more about these processes, experiments can be designed to understand when and how pathological changes begin, providing important clues toward developing interventions to prevent or treat disease. A great deal has been learned about structural and functional changes that occur in different body systems. Research has expanded our knowledge, too, of the biologic factors associated with extended longevity in humans and animal models.
This section of the NIA's narrative discusses some recent advances in the biology of aging. It begins with a Story of Discovery about new insights into the genetic and molecular basis of longevity, and several new advances in extending the lifespan. A discussion of promising new avenues of stem cell research follows, along with several other important NIA-supported findings from the past year.
Extending the Lifespan
In order to understand the aging process, it is important to identify those factors that affect the overall lifespan of an organism. Understanding the responsible physiological mechanisms and, further, identifying ways to slow down age-related changes are important. Beyond any gains in lifespan, studies in this area are aimed more importantly at developing interventions to keep older people healthy and free of disease and/or disability as long as possible. Experiments in a number of animal models are providing valuable insights into the mechanisms of longevity.
Story of Discovery: Genetic and Molecular Basis of Longevity Jeanne Calment of Arles, France is believed to have lived longer than any other person in recorded history. When she died on August 4, 1997, she was 122 years, 5 months, and 14 days old. What factors allowed her to live such a long life? Life expectancy in the United States has risen dramatically in the 20th century, from under 50 years in 1900 to about 73 for men and 79 for women in 1999; however, of the world's current 6 billion inhabitants, perhaps no more than 25 people are more than 110 years old. Today, scientists believe that while Madame Calment's exceptional longevity may be partially attributable to her lifestyle, her genetic makeup almost certainly played a substantial role. Increasingly, evidence points to a significant genetic influence on longevity. In one recent study of four families with a high number of members surviving to 90 years or more, researchers found evidence of a familial cluster of longevity that cannot be explained by chance alone. Researchers in a second study found that exceptionally long-lived people may pass on to their children lifelong protection against major diseases of aging, including an unusually good pattern of circulating cholesterol, a major factor affecting risk of cardiovascular disease. And in a third study, investigators studying centenarians or near-centenarians and their siblings identified a portion of chromosome 4 on which they believe a gene for exceptional longevity may be located. In fact, it is likely that heredity, environment, and lifestyle all have complex roles in determining a long and healthy life. But is there a maximum human lifespan beyond which we cannot live no matter how optimal our environment or favorable our genes? And perhaps, most importantly, how can insights into longevity be used to fight age-related diseases and disabilities to ensure a healthy, active, and independent life well into very old age? Since the 1930s, investigators have consistently found that laboratory rats and mice live up to 30 percent longer than usual when fed a diet that has at least 30 percent fewer calories than they would normally consume, but is nutritionally balanced. This was the first demonstration that the maximum lifespan of a mammal could be increased. More recent research has found that these animals also appear to be more resistant to age-related diseases including cancer. Other rodent studies have found that caloric restriction may increase resistance of neurons in the brain to dysfunction and death. In fact, caloric restriction appears to delay normal age-related degeneration of a number of physiological systems in rodents. Studies on the effects of caloric restriction in higher mammals (monkeys) are ongoing. Preliminary results are promising, including greater resistance to diabetes and heart disease in these animals. Yet even if caloric restriction is successful in extending primate lifespan, it is doubtful that it will ever become acceptable for most humans. However, caloric restriction shows that lifespan can be altered, prompting research into possible mechanisms. Why calorically restricted animals live longer and have reduced rates of age-related diseases is still unclear. Over the years, scientists have approached this question by identifying and characterizing genes that modify the lifespan of various organisms including yeast, fruit flies, worms, and mice to determine which biological pathways are involved in lifespan extension and to determine if these same pathways may be affected by caloric restriction or other interventions. At least 15 different life-extending genetic manipulations have been identified in the past ten years in these organisms. These genetic manipulations pinpoint three metabolic systems: the cellular response to stress, especially oxidative stress; hormonal control; and processes like metabolic rate that are altered by caloric restriction. Oxidative Stress. Oxidative stress, or damage to cells by metabolic by-products known as free radicals, is implicated in many of the processes of aging. Antioxidants, which can be nutrients such as vitamin E or compounds that are naturally produced within the body, combat oxidative stress by neutralizing free radicals. One particularly potent antioxidant is superoxide dismutase (SOD), an enzyme produced within the cell. High levels of anti-oxidants have been associated with longer lifespans in some model systems. Studies have shown that inserting extra copies of the gene for SOD production into fruit flies extends their average lifespan by as much as 30 percent, and researchers have found that giving C. elegans, a tiny worm with a very short lifespan, synthetic forms of antioxidants significantly extends their life. Interestingly, caloric restriction increases the resistance of organisms, including mice, to oxidative stress, again suggesting that there may be a relationship between stress resistance and aging. Hormonal control. A major breakthrough occurred in 1995 when researchers discovered that mutations in certain genes in C. elegans can also substantially extend its lifespan. One of these genes, called daf-2, controls a special stage in the worm's development called dauer formation, a metabolically slowed, nonaging state that it enters when food is limited or there is overcrowding. Other investigators have detected mutations in similar daf genes that increase lifespan three-or even four-fold. These genes are similar in structure to genes in humans for a protein that binds the hormone insulin to cells and for an enzyme involved in causing cellular changes in response—the so-called IGF-1 signaling pathway. The similarities suggest that worms also have an IGF-1-like signaling pathway, and that reducing its activity may increase their lifespan. In the late 1990s, researchers discovered that fruit flies also have such a pathway, and that mutations in the genes for this pathway also extend fruit fly lifespan. Around the same time, a study showed a possible relationship between IGF-1 activity and longevity in mice. Dwarf mice have low levels of several hormones, including growth hormone, which normally stimulates production of IGF-1. These mice have low levels of IGF-1 and are also long-lived. Scientists recently found that mutations that stop growth hormone function in mice not only increase lifespan, but also delay the point at which the cell permanently stops dividing, suggesting an effect on the rate of aging as well as on lifespan. These results highlight the important influence of hormonal regulation on aging. Metabolic Rate. A mutation in a gene affecting metabolism also increases lifespan in fruit flies. This mutation affects a protein that carries metabolic products of carbohydrates and fats called dicarboxylic acids into the energy factories of the cell, the mitochondria, where the dicarboxylic acids are converted into chemical energy. In flies with this mutation, the mitochondria have less access to dicarboxylic acid fuels and therefore lowered energy production, and the flies' life span is increased. This result may be a clue to one mechanism extending lifespan by calorie restriction as it is likely that caloric restriction would similarly restrict fuel available to mitochondria for conversion into energy. Implications for Human Aging. The genes isolated so far in model systems are only a few of what scientists think may be dozens, perhaps hundreds, of longevity-and aging-related genes active in many different body pathways. The next big question is whether counterparts in people—human homologs—of the genes found in laboratory animals have similar effects. If they do, these ultimately could yield clues about how genes interact with environmental factors to influence longevity in humans. The outcome of this ongoing exploration of genetic and non-genetic factors affecting lifespan has been to show that aging is not as immutable as previously supposed, and that we may eventually be able to identify practical ways of extending active and healthy lifespan in humans. |
The Promise of Stem Cell Research
Human pluripotent stem cells—that is, cells that are capable of dividing for indefinite periods in culture and of giving rise to most tissues of an organism—hold enormous potential for cell replacement or tissue repair therapy in many degenerative diseases of aging. For disorders affecting the nervous system, such as Alzheimer's and Parkinson's diseases, amyotrophic lateral sclerosis, and spinal cord and brain injury, transplantation of neural cell types derived from human pluripotent stem cells offers the potential of replacing cells lost in these conditions and of recovery of function. Human pluripotent stem cells can also provide a model for studying fundamental molecular and cellular processes important in understanding aging and age-related diseases. Another type of stem cell, multipotent or "adult" stem cells, are committed to producing cells that have a particular function. For example, stem cells circulating in the blood give rise to red blood cells, white blood cells, and platelets, but not bone cells or liver cells. Until recently, there was little evidence in mammals that multipotent cells could "change course" and produce cells of a different type. However, recent findings suggest that under certain conditions, some adult stem cells previously thought to be committed to the development of one line of specialized cells are able to develop into other types of specialized cells. Neural stem cells are of particular interest to the study of AD and other neurodegenerative diseases of aging. Through several recent studies, we have found that environmental cues, which vary among brain subregions, may determine the fate of a stem cell, that neurogenesis may require the cooperation of multiple protein factors, and that neural stem-like cells taken from post-mortem brain tissue can form neurons. Together, these studies continue to show the potential of adult-derived neural stem cells to make different kinds of brain cells.
Stem Cells Help Repair Damaged Heart and Brain. In the mouse, stem cells show potential to replace cells lost in either the heart or brain. When primitive bone marrow cells (a type of stem cell) are injected into the mouse circulatory system, they can find their way to the damaged brain and gradually change into neuronal cells. When bone marrow cells are transplanted into mouse hearts damaged by a "heart attack," these cells regenerate not only new heart muscle but also blood vessel components. In mice, this repair can be accomplished in just a few weeks. In a recent, highly provocative study, mice in which heart damage had been induced were injected with cytokines (proteins) called stem cell factor (SCF) and granulocyte-colony-stimulating factor (G-CSF). Stimulated by the cytokines, primitive bone marrow cells swarmed to the hearts, converted to several different types of cardiac cells, and contributed to repair of the damaged tissue, improving both the heart function and the survival of the treated mice. This finding, while preliminary, suggests that it may be possible to mobilize the body's own naturally-occurring stem cells to repair tissue damage and fight disease.
Gene Expression and Aging
Characterization and Functional Classification of 15,000 Mouse Genes. New technologies are providing answers to questions about how genes control cell and tissue function. Arrays of DNA for specific genes permit the comparison of tens of thousands of genes at one time, to determine which are turned on or off in a particular cell or condition. A collection of 15,000 mouse genes has been developed, with emphasis on inclusion of genes active in placenta and embryo development. To facilitate extensive use of this gene collection, the set (currently named the "NIA mouse 15K cloned gene set") has been made available as a resource to the scientific community. This collection has been distributed to more than 100 research institutions world-wide. Nearly complete sequences of each gene in the 15K gene set are also available; by comparing the sequence information with genes that have already been well studied, scientists may be able to determine the function of these genes in mice. The Institute has also developed the NIA Microarray Facility, which provides investigators with low-cost access to microarrays developed from the set and will also provide for collecting and analyzing the gene expression findings of multiple investigators.
Gene Required for Full Reproductive Lifespan in Women. One to three percent of women have premature ovarian failure (POF), going through menopause before age 40. In a number of these cases, a mutant gene is likely to be the cause, but until now no gene directly involved in regulating the time of menopause in women has been identified. Recently, researchers isolated a gene, FOXL2, that is mutated in this condition. FOXL2 is required to activate a number of other genes in the ovary. When the function of FOXL2 is reduced by a mutation, the number of follicles (eggs) in the ovary falls to a level too low to sustain a full reproductive lifespan. These findings reveal the first gene that is critically involved in determining the number of follicles in a woman's ovary; as more is learned about FOXL2's function, interventions that prevent or alleviate POF may be developed. In addition, an understanding of the genes that affect premature menopause will help in understanding the normal menopause process and its consequences.
Behavioral and Social Research
Behavioral and lifestyle factors have a profound impact on health throughout the lifespan.
Older adults can help to prevent disease and disability and improve their quality of life through healthy behaviors such as proper nutrition, exercise, use of preventive health care, and avoiding smoking and alcohol abuse. Several particularly encouraging studies have shown that disability rates are declining, and NIA research is focusing on ways to sustain and even accelerate the decline in disability, including the use of behavioral interventions and the health care system by older people. In addition, important research efforts, such as the national Health and Retirement Study, continue to collect and analyze demographic data that inform public policy and planning for the health, economic, and social needs of a growing older population.
Potential Impact of Attitudes on Health and Behavior
Emotional state has been associated with health and functional status in old age. Both positive and negative attitudes or emotions can influence health and physical and cognitive function.
Positive Emotions in Early Life Linked to Longevity. Findings from the Nun Study, a longitudinal study of Alzheimer's disease and aging that follows the lives of older members of a religious order in the United States, indicate that positive emotional content in early life autobiographies was strongly associated with longevity six decades later. Nuns who expressed more positive emotions in their autobiographies lived significantly longer than nuns expressing fewer positive emotions. Finding such a strong association between written positive emotional expression and longevity indicates a need for research that sheds light on the underlying mechanisms responsible for and associated with this relationship.
Personality Determinants of HIV Risk Perceptions and Behavior Changes. Eleven percent of people with AIDS in the United States are over age 50. A recent study conducted in an economically disadvantaged and high-risk group suggested that personality traits are associated with perceptions of risk for HIV. The investigators also found that individuals who believed they were at high risk for being infected with HIV were no more likely to increase condom use after a four-session risk reduction intervention than those who thought they were at low risk. However, individuals who showed greater conscientiousness and those who had a stronger sense of their own competence and ability to control their own behavior were more likely to adopt condom use. These findings provide insight into high-risk sexual behavior among older Americans and may help us develop interventions that modulate them.
Physical and Cognitive Disability Continue To Decline Among American Elders. When scientists assess disability in the population, they may look at a number of factors. One is the extent to which individuals can conduct basic activities of daily living such as eating, dressing, or bathing or participate in routine care activities such as everyday household chores or managing money. Scientists also track the extent to which cognitive disabilities such as memory loss are present in the population. Recent studies have indicated that both physical and cognitive decline are decreasing among the elderly in the United States. The 1999 National Long Term Care Survey (NLTCS), the latest of a series of surveys of the elderly population (particularly those who are functionally impaired), continues to document a dramatic decline in the overall prevalence of physical disability among older Americans over the past two decades. While 26.2 percent of the elderly were assessed as disabled in 1982, this figure dropped to 19.7 percent in 1999. In addition, successive iterations of the NLTCS suggest that the rate of disability decline may be accelerating. Of particularly note is the sharp—and accelerating—reduction in disability rates among African Americans during the 1990s, reversing trends from the 80s. Results from the 1999 NLTCS also show large declines in severe cognitive impairments, with 900,000 fewer cases in 1999 than expected based on the 1982 rates—a decline in prevalence from 5.2 to 2.7 percent. The finding that cognitive disability is declining is supported by evidence from the Health and Retirement Study, a major national study of the lives of older Americans. In this study, declines were especially large among those with less than a high school education and those ages 80 and older. Cognitive decline is most prevalent in the oldest old, the segment of the population projected to grow most rapidly in coming years. Thus, if current rates of cognitive impairment persist, the actual number afflicted will increase dramatically. A decline in the percentage of cognitively impaired would have an important effect in countering this trend. These findings are potentially of great significance in identifying and addressing causes of disability, as well as informing national health care policy. Declining rates of chronic disability may also moderate the burden of caregiving, including the informal care provided within families, the care provided through home health services, and the care provided in long-term care institutions. Most importantly, they indicate that elderly Americans are more likely than ever to enjoy the robust health and independence that characterize a life free of chronic physical or cognitive disability. |
Health Disparities
The health status of racial and ethnic minority groups in the U. S. has improved steadily over the last century. Despite such progress, disturbing disparities in health persist between majority and minority populations. In 1997, for example, average life expectancy at age 65 was 16.1 years for African Americans and 17.8 years for Caucasians. Demographic projections predict a substantial change in the racial and ethnic makeup of the older population, heightening the need to examine and reduce differences in health and life expectancy. Research to date has shown that health disparities are associated with a broad, complex, and interrelated array of factors. Disease risk, diagnosis, progression, response to treatment, caregiving, access to care, and overall quality of life each may be affected by variables such as race, ethnicity, gender, socioeconomic status, age, education, occupation, country of origin, and possibly other lifetime and lifestyle differences. The NIA is committed to addressing health disparities through its research programs. For example, Satellite Diagnostic and Treatment Centers, part of the national Alzheimer's Disease Centers (ADC) Program, have successfully recruited African Americans, Hispanics, Native Americans, and American Indian/Alaska Natives to AD prevention and treatment studies. Researchers on the NIA's Religious Orders Study have made a major effort to enroll African American members of the Catholic clergy; the nature of the study population enables the etiology and pathology of AD to be established among individuals with similar educations, occupations, socioeconomic status, and lifestyles. Five ADCs received funding in 2000 and 2001 specifically to encourage minority-related research, and in 2001 half of the NIA Director's Reserve funds, which encourage collaborative research projects, were allocated to minority-focused research. In addition, the NIA recently completed a year-long review of these issues and developed a comprehensive strategic plan to address health disparities in the older population.
Racial Differences in Cognitive Performance in Elders Disappear When Quality of Education Is Assessed. Racial comparisons on intelligence tests, neuropsychological tests, cognitive tests, and dementia batteries generally have shown that despite equating the groups on variables such as years of education and socioeconomic status, African Americans score lower on these measures than Caucasians. In this study, investigators reassessed the results of a standard neuropsychological test battery using an estimate of quality of education, the individual's score on the Reading Recognition subtest from the Wide Range Achievement Test (WRAT). After adjusting for this score, the majority of previously noted test score differences between African Americans and Caucasians became non-significant. This finding indicates a factor that can account for ethnic group differences on cognitive tests, and suggests characteristics that can be incorporated into new cognitive tests and measures that are culturally fair.
More African-Americans Than Africans Get Alzheimer's Disease. Over a 5-year period, researchers followed 2,147 African-Americans in Indianapolis, Indiana, and 2,459 Yoruba in Ibadan, Nigeria, age 65 and older, to see if they developed dementia and AD. They used a screening instrument that they developed specifically for use in comparative epidemiological studies of dementia in culturally disparate non-literate and literate populations. All participants at both sites received the same examination, which included an interview, neuropsychological testing, examination by a physician, and laboratory and imaging studies, when deemed clinically appropriate. Great care was taken to ensure that diagnostic consistency was maintained within and between sites. The results indicated that in the African-American group, 3.24 percent per year developed dementia, including 2.52 percent per year who developed AD. In the African group, 1.35 percent per year developed dementia, including 1.15 percent per year who developed AD. The identification of populations in which the prevalence of AD is much lower or much higher than that in the United States may greatly facilitate our understanding of the disease's etiology.
Future Research Directions in Health Disparities
Resource Centers for Minority Aging Research (RCMARs). The NIA-supported Resource Centers for Minority Aging Research continue to represent one of the Institute's most visible and focused efforts to build the national research infrastructure for minority aging research. The six RCMARs maintain active involvement in activities addressing the original RCMAR mission of establishing a research mentoring mechanism in minority health, enhancing professional diversity in minority health research, evaluating/developing measurement tools tailored to minority populations, and developing strategies for recruiting and retaining minority research participants. The RCMAR program is scheduled for renewal in FY 2002. To increase the impact of research on the minority community, the second generation of RCMARs will have a stronger central theme at each Center, will further emphasize mentoring responsibilities, and will integrate their activities with those of other NIA-supported Center programs.
Conclusion: Meeting New Challenges Through Aging Research
At the beginning of this document, we told you about a few of the millions of healthy, productive Americans over 65. In stark contrast to just a century ago, the good health, productivity, and high quality of life enjoyed by Jimmy Carter, Madeleine L'Engle, Johnny Kelley, and Julia Child are now shared by large numbers of older men and women. Yet important work remains to be done. As our population rapidly grows older, it is ever more urgent that we find effective ways to address the often devastating diseases and conditions associated with advanced age. Since the NIA's founding in 1974, groundwork has been laid for today's important advances in understanding basic aging, preventing disease and disability, including Alzheimer's disease, and defining special social and behavioral issues for older individuals, their families and caregivers, and clinicians. The latest studies provide additional basic understandings as well as improved interventions to treat and even prevent some of the more devastating and disabling aspects of aging. With such research continued and intensified, we can move forward in meeting the promise of extended life by improving the health and well being of older people in America.
Budget Policy
The Fiscal Year 2003 budget request for the NIA is $971,709,000 including AIDS, an increase of $75,569,000 and 8.4 percent over the FY 2002 level.
A five year history of FTEs and Funding
FTEs by Fiscal Year  |
Funding Levels by Fiscal Year 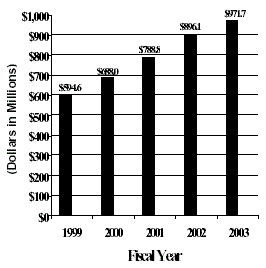
|
One of NIH's highest priorities is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. The Fiscal Year 2003 request provides average cost increases for competing RPGs equal to the Biomedical Research and Development Price Index (BRDPI), estimated at 4.0 percent. Noncompeting RPGs will be funded at committed levels which include increases of 3 percent on average for recurring direct costs. Future promises for advancement in medical research rest in part with new investigators with new ideas. In the Fiscal Year 2003 request, NIA will support 584 pre-and postdoctoral trainees in full-time training positions, the same number as in FY 2002. Stipend levels for NRSA trainees will increase by 4 percent over Fiscal Year 2002 levels.
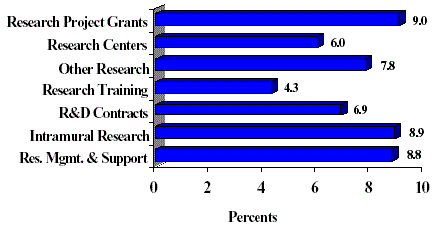
The Fiscal Year 2003 request includes funding for 68 research centers, 225 other research grants, including 192 clinical career awards, and 78 R& D contracts. The R& D contracts mechanism also includes support for 24 contracts for the Extramural Clinical and Pediatric Loan Repayment Programs. Intramural Research and Research Management and Support receive increases of 9 percent over FY 2002.
The Mechanism distribution by dollars and percent changes are displayed below:
FY 2003 Budget Mechanism (Dollars in Millions)

FY 2003 Estimate Percent Change From FY 2002 Mechanism
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
- Manton KG et al. Chronic disability trends in elderly United States populations: 1982-1994. Proc Nat Acad Sci USA 94: 2593-2598, 1997.
- Evans DA et al. Prevalence of Alzheimer's disease in a community population of older persons; higher than previously reported. JAMA 262: 2551-2556, 1989.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
- Small, G et al. Diagnosis and treatment of Alzheimer disease and related disorders. JAMA 16: 1363-1371, 1997.
- Ernst, RL, et al. Cognitive function and the costs of Alzheimer's disease, Arch Neurol 54: 687-693, 1997.
- National Center for Health Statistics. Health, United States, 1999 With Health and Aging Chartbook. Figure 11, pg. 41. Hyattsville, MD: 1999.
- Marcantonio ER et al. Reducing delirium after hip fracture: a randomized trial, J. Am Geriatr Soc 49: 516-522, 2001.
- American Cancer Society. Cancer Facts and Figures. 2001.
- Coulam CB et al. Incidence of premature ovarian failure. Obstetrics and Gynecology 67: 604-606, 1986.
- AIDS Among Persons Aged $ 50 Years --United States, 1991-1996. MMWR 47: 21-27, 1998.
