 No, don't take us literally! Humans cannot drink saline water. But, saline water can be made into freshwater, which everyone needs everyday. The process is called desalination, and it is being used more and more around the world to provide people with needed freshwater. Most of the United States has, or can gain access to, ample supplies of fresh
water for drinking purposes. But, fresh water can be in short supply
in some parts of the country (and world). And, as the population continues to grow,
shortages of fresh water will occur more often, if only in certain
locations. In some areas, salt water (from the ocean, for instance) is being
turned into freshwater for drinking.
No, don't take us literally! Humans cannot drink saline water. But, saline water can be made into freshwater, which everyone needs everyday. The process is called desalination, and it is being used more and more around the world to provide people with needed freshwater. Most of the United States has, or can gain access to, ample supplies of fresh
water for drinking purposes. But, fresh water can be in short supply
in some parts of the country (and world). And, as the population continues to grow,
shortages of fresh water will occur more often, if only in certain
locations. In some areas, salt water (from the ocean, for instance) is being
turned into freshwater for drinking.
A promising method to desalinate seawater is the "reverse osmosis" method. Right now, the high cost of desalinization has kept it from being used more often, as it can cost over $1,000 per acre-foot to desalinate seawater as compared to about $200 per acre-foot for water from normal supply sources. Desalinization technology is improving and costs are falling, though, and Tampa Bay, FL is currently desalinizing water at a cost of only $650 per acre foot. As both the demand for fresh water and technology increase, you can expect to see more desalinization occurring, especially in areas, such as California and the Middle East.
What do we mean by "saline water?" Water that is saline contains significant amounts (referred to as "concentrations") of dissolved salts. In this case, the concentration is the amount (by weight) of salt in water, as expressed in "parts per million" (ppm). If water has a concentration of 10,000 ppm of dissolved salts, then one percent (10,000 divided by 1,000,000) of the weight of the water comes from dissolved salts.
Here are our parameters for saline water:
![]() Fresh water - Less than 1,000 ppm
Fresh water - Less than 1,000 ppm
![]() Slightly saline water - From 1,000 ppm to 3,000 ppm
Slightly saline water - From 1,000 ppm to 3,000 ppm
![]() Moderatly saline water - From 3,000 ppm to 10,000 ppm
Moderatly saline water - From 3,000 ppm to 10,000 ppm
![]() Highly saline water - From 10,000 ppm to 35,000 ppm
Highly saline water - From 10,000 ppm to 35,000 ppm
By the way, ocean water contains about 35,000 ppm of salt.
 The scarcity of fresh water resources and the need for additional water supplies is already critical in many arid regions of the world and will be increasingly important in the future. It is very likely that the water issue will be considered, like fossil energy resources, to be one of the determining factors of world stability. Many arid areas simply do not have fresh water resources in the form of surface water such as rivers, lakes, etc. and have only limited underground water resources that are becoming more brackish as abstraction of water from the aquifers continues. The world-wide availability of renewable energies and the availability of mature technologies in this field make it possible to consider the coupling of desalination plants with renewable energy production processes in order to ensure the production of water in a sustainable and environmentally friendly scheme for the regions concerned. Solar desalination is used by nature to produce rain which is the main source of fresh water on earth. All available man-made distillation systems are a duplication on a small scale of this natural process. Recently, considerable attention has been given to the use of renewable energy as sources for desalination, especially in remote areas and islands, because of the high costs of fossil fuels, difficulties in obtaining it, attempts to conserve fossil fuels, interest in reducing air pollution, and the lack of electrical power in remote areas.
The scarcity of fresh water resources and the need for additional water supplies is already critical in many arid regions of the world and will be increasingly important in the future. It is very likely that the water issue will be considered, like fossil energy resources, to be one of the determining factors of world stability. Many arid areas simply do not have fresh water resources in the form of surface water such as rivers, lakes, etc. and have only limited underground water resources that are becoming more brackish as abstraction of water from the aquifers continues. The world-wide availability of renewable energies and the availability of mature technologies in this field make it possible to consider the coupling of desalination plants with renewable energy production processes in order to ensure the production of water in a sustainable and environmentally friendly scheme for the regions concerned. Solar desalination is used by nature to produce rain which is the main source of fresh water on earth. All available man-made distillation systems are a duplication on a small scale of this natural process. Recently, considerable attention has been given to the use of renewable energy as sources for desalination, especially in remote areas and islands, because of the high costs of fossil fuels, difficulties in obtaining it, attempts to conserve fossil fuels, interest in reducing air pollution, and the lack of electrical power in remote areas.
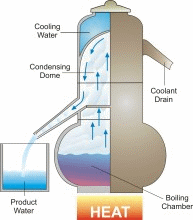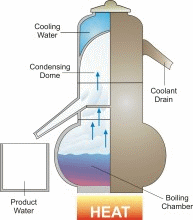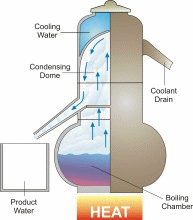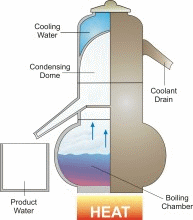 Desalination/Distillation is one of mankind's earliest forms of water treatment, and it is still a popular treatment solution throughout the world today. In ancient times, many civilizations used this process on their ships to convert sea water into drinking water. Today, desalination plants are used to convert sea water to drinking water on ships and in many arid regions of the world, and to treat water in other areas that is fouled by natural and unnatural contaminants. Distillation is perhaps the one water treatment technology that most completely reduces the widest range of drinking water contaminants.
Desalination/Distillation is one of mankind's earliest forms of water treatment, and it is still a popular treatment solution throughout the world today. In ancient times, many civilizations used this process on their ships to convert sea water into drinking water. Today, desalination plants are used to convert sea water to drinking water on ships and in many arid regions of the world, and to treat water in other areas that is fouled by natural and unnatural contaminants. Distillation is perhaps the one water treatment technology that most completely reduces the widest range of drinking water contaminants.
In nature, this basic process is responsible for the hydrologic cycle. The sun causes water to evaporate from surface sources such as lakes, oceans, and streams. The water vapor eventually comes in contact with cooler air, where it re-condenses to form dew or rain. This process can be imitated artificially, and more rapidly than in nature, using alternative sources of heating and cooling.
The above diagram and information is courtesy of Desware: The Encylopedia of Desalination and Water Resources.
Some of this information came from the Water Education Foundation and from the Corpus Cristi TAMU-CC Public Administration.
In California, USA, the towns of Santa Barbara and Avalon have begun using desalination methods to remove the salt from seawater and make it suitable for drinking. A promising method to desalinate seawater is the "reverse osmosis" method. You might be more familiar with another method, distillation, which involves heating seawater and capturing and condensing the steam. As of 1998, the high cost of desalination has kept it from being used more often, as it can cost over $1,000 - $2,200 per acre-foot (1992 cost basis) to desalinate seawater as compared to about $200 per acre-foot for water from normal supply sources. Desalination technology is improving and costs are falling, though, and Tampa Bay, Florida is currently desalinizing water at a cost of only $650 per acre foot. As both the demand for freshwater and technology increase, you can expect to see more desalination occurring, especially in areas, such as California and the Middle East.