
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-193 - Reserpine
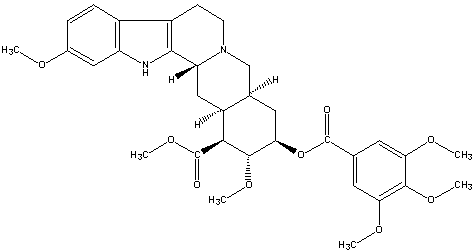
| Chemical Formula: C33H40N2O9 | - | 3D Structure* | |
|---|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | |||
A bioassay for possible carcinogenicity of reserpine, an antihypertensive drug for human use, was conducted by administering the test chemical in feed to F344 rats and B6C3F1 mice.
Groups of 50 rats and 50 mice of each sex were administered reserpine at two doses, 5 ppm and 10 ppm, for 103 weeks and then observed for an additional 2 weeks. Matched controls consisted of groups of 50 untreated rats and 50 untreated mice of each sex. All surviving animals were killed and necropsied at the end of 104 or 105 weeks.
The significant effects that could be related to administration of reserpine at the doses used were decreased body weight and increased tumor formation in dosed male rats and in mice of both sexes. Dosed male rats had an increased incidence of adrenal medullary pheochromocytomas. Among dosed mice, some males developed undifferentiatedcarcinomas of the seminal vesicles, which rarely occur in control mice, and females had an increased incidence of mammary cancer.
It was concluded that, under the conditions of the bioassay, reserpine was carcinogenic in male rats and in mice of both sexes, producing three different kinds of cancers. reserpine was not carcinogenic for female rats, but they may not have received a high enough dose for maximum test sensitivity.
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Positive | |
| Female Rats: | Negative | |
| Male Mice: | Positive | |
| Female Mice: | Positive | |
Report Date: November 1982
Target Organs from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.