
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-183 - Dibutyltin Diacetate
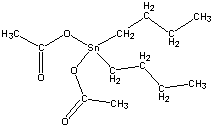
| Chemical Formula: C12H24O4Sn | - | 3D Structure* | |
|---|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | |||
Dibutyltin diacetate, a widely used catalyst for polymerization reactions, was selected for bioassay by the National Cancer Institute in an effort to screen a number of organo-metallic compounds for carcinogenicity.
A bioassay for the possible carcinogenicity of dibutyltin diacetate was conducted using Fischer 344 rats and B6C3F1 mice. Dibutyltin diacetate was administered in the feed, at either of two concentrations, to groups of 50 male and female animals of each species. Twenty animals of each sex and species were placed on test as controls. The high and low time-weighted average dietary concentrations of dibutyltin diacetate were, respectively, 133 and 66.5 ppm for rats and 152 and 76 ppm for mice. The compound was administered for 78 weeks to rats and mice, followed by a period of no compound administration of 26 weeks for rats and 14 weeks for mice.
There were significant positive associations between the concentrations of dibutyltin diacetate administered and mortality in male rats and female mice. There were no significant positive associations between the concentrations administered and mortality in female rats or male mice. Adequate numbers of animals in all groups survived sufficiently long to be at risk from late-developing tumors. Mean body weight depression, relative to controls, was observed in male mice and significantly accelerated mortality, relative to controls, was observed in male rats and female mice, indicating that the concentrations of dibutyltin diacetate administered to these animals may have approximated the maximum tolerated concentrations. Since no mean body weight depression, no significantly accelerated mortality, and no other signs of toxicity were associated with administration of dibutyltin acetate to femalerats, it is possible that these animals may have been able to tolerate a higher dietary concentration.
There were no neoplasms occurring in statistically significant higher incidences in dosed rats or mice when compared to their respective controls. However, there was an accidental loss of tissues from high dose female rats which precluded an evaluation of carcinogenicity in this group of animals. There was a significant positive association between the concentrations administered and the incidences of hepatocellular adenomas in females mice; however, the Fisher exact comparisons were not significant using the Bonferroni criterion. Liver neoplasms (i.e., a combination of adenomas and carcinomas) were also observed in male mice; however, the occurrence was not statistically significant.
Under the conditions of this bioassay, there was no conclusive evidence for the carcinogenicity of dibutyltin diacetate in male Fischer 344 rats or B6C3F1 mice of either sex. The loss of tissues taken from high dose female rats in this bioassay precluded an evaluation of the carcinogenicity of dibutyltin diacetate to female Fischer 344 rats.
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Negative | |
| Female Rats: | Inadequate Study | |
| Male Mice: | Negative | |
| Female Mice: | Negative | |
Synonyms: bis(acetyloxy)dibutylstannae; diacetoxydibutylstannae; diacetoxybutyltin; dibutyl tin diacetate
Report Date: 1979
Target Organs from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.