 |

A Message from NCI Director Dr. Andrew C. von Eschenbach
In April, while testifying before the Senate Appropriations Subcommittee on Labor, Senator Arlen Specter (R-Pa.) asked me an important question: What would it take to accelerate by 5 years the achievement of the 2015 goal of eliminating the suffering and death due to cancer? I want to share the National Cancer Institute's response to Senator Specter so that the cancer community can better understand what we hope to achieve and how we hope to achieve it.
You have requested information on the amount of money necessary for the National Cancer Institute (NCI) to achieve its 2015 goal by 2010. It should be noted, though, that these funding estimates for additional resources were developed without taking into consideration overall fiscal constraints and other competing priorities of the National Institutes of Health (NIH), the Department of Health and Human Services (HHS), or the rest of the Federal government over this 5-year time period. The current annual NCI budget is nearly $5 billion and the resources discussed below would be in addition to this base.
NCI has established an ambitious goal of eliminating the suffering and death due to cancer by 2015 by sustaining and integrating progress in the discovery, development, and delivery of more effective interventions based on molecular mechanisms of cancer. We estimate that expenditure of an additional $4.2 billion above the NCI base of nearly $5 billion over the next 5 years could accelerate progress. While the elimination of suffering and death due to cancer may not be fully achievable by 2010, there would be significant progress toward narrowing the gap between 2015 and 2010.
This $4.2 billion estimate reflects an additional upfront allocation of $2.5 billion to be expended over 5 years for a National Advanced Technology Initiative for cancer (NATIc) to accelerate the emerging disciplines of molecular oncology, nanotechnology, and bioinformatics for use in creating a pipeline of new personalized cancer diagnostics and therapeutics. This would also reflect an annual increase of $171 million over current base NCI levels for 5 years to deploy a modern integrated cancer clinical trials infrastructure and an annual increase of $164 million for 5 years to expand and integrate the NCI-designated Cancer Centers program from 60 existing centers to 75. In addition to resources, additional legislative authorities related to exemptions from specific parts of current procurement, grant review and processing, and licensing and patenting rules would also help speed progress toward an accelerated cancer goal.
Read more 

 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

A Message from NCI Director Dr. Andrew C. von Eschenbach
In April, while testifying before the Senate Appropriations Subcommittee on Labor, Senator Arlen Specter (R-Pa.) asked me an important question: What would it take to accelerate by 5 years the achievement of the 2015 goal of eliminating the suffering and death due to cancer? I want to share the National Cancer Institute's response to Senator Specter so that the cancer community can better understand what we hope to achieve and how we hope to achieve it.
You have requested information on the amount of money necessary for the National Cancer Institute (NCI) to achieve its 2015 goal by 2010. It should be noted, though, that these funding estimates for additional resources were developed without taking into consideration overall fiscal constraints and other competing priorities of the National Institutes of Health (NIH), the Department of Health and Human Services (HHS), or the rest of the Federal government over this 5-year time period. The current annual NCI budget is nearly $5 billion and the resources discussed below would be in addition to this base.
NCI has established an ambitious goal of eliminating the suffering and death due to cancer by 2015 by sustaining and integrating progress in the discovery, development, and delivery of more effective interventions based on molecular mechanisms of cancer. We estimate that expenditure of an additional $4.2 billion above the NCI base of nearly $5 billion over the next 5 years could accelerate progress. While the elimination of suffering and death due to cancer may not be fully achievable by 2010, there would be significant progress toward narrowing the gap between 2015 and 2010.
This $4.2 billion estimate reflects an additional upfront allocation of $2.5 billion to be expended over 5 years for a National Advanced Technology Initiative for cancer (NATIc) to accelerate the emerging disciplines of molecular oncology, nanotechnology, and bioinformatics for use in creating a pipeline of new personalized cancer diagnostics and therapeutics. This would also reflect an annual increase of $171 million over current base NCI levels for 5 years to deploy a modern integrated cancer clinical trials infrastructure and an annual increase of $164 million for 5 years to expand and integrate the NCI-designated Cancer Centers program from 60 existing centers to 75. In addition to resources, additional legislative authorities related to exemptions from specific parts of current procurement, grant review and processing, and licensing and patenting rules would also help speed progress toward an accelerated cancer goal.
Three decades ago there were 3 million U.S. cancer survivors; today that number has increased to over 10 million. Today, each minute of every hour of every day, 1 American dies from cancer: 570,280 lives will be lost this year due to this disease. Despite this fact, there has been remarkable progress in understanding the cancer process and applying that knowledge. Today, 65 percent of patients diagnosed with cancer can expect to survive. If we had the ability to apply what we know today to every cancer patient, we could have an immediate impact on survival, largely through the NCI Cancer Centers. Incremental improvements in survival will continue toward our 2015 goal, but we can accelerate these gains. Even improving the overall survival rate to 90 percent by 2010 could mean an additional 850,000 lives saved. The impact of this strategy could produce annual changes in the first 2 years of around 2 to 3 percent with larger increases occurring in 2008-10.
For most cancer patients, survival is greatly influenced by early detection. The rapid deployment of advanced imaging, nanotechnology-supported early detection platforms, and targeted therapies will change the face of diseases such as ovarian, lung, colon, and breast cancers, where survival is low because we can not currently detect them before they spread. Ovarian cancer, which is very difficult to detect and diagnose in its early stages, has over 25,000 new cases diagnosed annually and over 14,000 deaths; the mortality rate is nearly 85 percent. Imaging and detection techniques presently under development and broadly applied could reverse that mortality rate to be an 85 percent survival rate. Lung cancer, with approximately 170,000 expected deaths this year, would see a significant reduction in the number of deaths if the application of new technologies combined with other interventions could be universally applied in an accelerated manner.
The challenge to achieving the goal of eliminating the suffering and death due to cancer by 2010 is daunting, but with the authorities and appropriations commensurate with the task, the pace of progress could be accelerated, and the gap between 2015 and 2010 narrowed. The following reflects a brief overview of how such funds, if available, could be applied.
- Rapid Deployment of a National Advanced Technology Initiative for Cancer: $2.5 billion one-time appropriation with commensurate authorities.
- Deployment of a Modern Integrated Clinical Trials Infrastructure: $171 million addition to the NCI base budget.
- Expansion and Integration of the Cancer Centers Program: $164 million addition to the NCI base budget.
- Mechanisms and Flexibilities: streamlined procurement and review processes to acquire materials and services; coordination of licensing and patenting activities.
A National Advanced Technology Initiative for cancer (NATIc) could provide a linkage between the National Cancer Program and research and development (R&D) initiatives being developed in selected national laboratories and advanced technology facilities located in more than 40 states and regions. Connected in real time through a common bioinformatics grid, NATIc could serve as a "network of networks" of science, technology, and treatment to accelerate the emerging discipline of molecular oncology to create a pipeline of new personalized cancer diagnostics and therapeutics from bench concept to bedside and community delivery. In the next few years, such an initiative could:
- Accelerate the implementation of a nationwide high-end information technology grid for bioinformatics that could be uniquely adapted for real-time data sharing. NCI's pilot version, the Cancer Biomedical Informatics Grid, called caBIG, is currently being implemented among 50 cancer centers, the Food and Drug Administration (FDA), and other organizations.
- Develop a comprehensive biomarker discovery and validation program.
- Foster the application of emerging technologies, such as nanotechnology, and integrate molecular agents with advanced imaging devices.
- Accelerate a nationwide "real-time" medical information electronic system for research and medical data sharing using technologies and devices currently employed by the banking industry and large-scale commercial enterprises.
- Enhance the discovery and validation of new targets of genes and proteins critical to cancer development.
NCI could deploy a more modern and integrated infrastructure for cancer clinical trials. This clinical research infrastructure could:
- Strengthen collaborations with industry, FDA, the Centers for Medicare and Medicaid Services, and other public, private, academic, and patient advocacy organizations to oversee the conduct of cancer clinical trials.
- Develop new infrastructure and procedures to standardize, coordinate, and track clinical trials development and accrual across all NCI-supported clinical trials.
- Increase utilization of imaging tools in screening and therapy trials, evaluate new imaging probes and methodologies, enable access to the imaging data from trials in an electronic format, and facilitate evaluation of image-guided interventions.
- Expand access and improve the timeliness for completion of the highest priority clinical studies.
- Foster the development of a cadre of established clinical investigators who could work between bench and bedside.
- Pilot new approaches and develop prototypes for clinical trials networks that could improve the efficiency, coordination, and integration of our national efforts.
- Develop a common clinical trials informatics platform that could be made available to the full range of investigators working within the cancer clinical trials system.
NCI could accelerate the expansion and integration of the NCI-designated Cancer Centers program, including the addition of 15 new cancer centers, increasing the number of centers from the current 60 to 75. The Cancer Centers program could:
- Implement progressive bioinformatics and communication systems to achieve horizontal integration.
- Fund additive programs in collaborative, multidisciplinary research and require integration and sharing of results.
- Broaden the geographic impact of the centers, networks, and consortia and vertically integrate them with community and regional health care delivery systems.
- Improve the access of minority and underserved populations to state-of-the-art research and resources.
- Create and strengthen partnerships with government agencies and community organizations.
- Broadly provide expertise and other resources to caregivers, patients and families, and appropriate health agencies.
In addition to appropriations, flexible legislative authorities related to exemptions from specific parts of current procurement, grant review and processing, and licensing and patenting rules could also help accelerate progress. A streamlined procurement process could facilitate the acquisition of materials and services to support the R&D activities. Technology development could also be enhanced by sufficient flexibility and integration to enable interactions among a wide array of laboratories and other entities. Expedited review procedures and work flow processing could help to award funds in sequence as needed. This might include direct solicitation from known laboratories or other sources of technology, and the capability to terminate funding instruments at the convenience of the government with limited appeal processes so that funds could be redirected from low-performing consortia to more productive venues.
Coordination of the licensing and patenting activities among grantees, contractors, and the intramural program could also be useful for many of the multicomponent technology platforms that could be created through this effort. An accelerated process for Determination of Exceptional Circumstances (DEC) and deviations from appropriate Federal Acquisition Regulation (FAR) clauses, when deemed valuable to the broad research enterprise, could be utilized.
Dr. Andrew C. von Eschenbach
Director, National Cancer Institute
|
 |
 |

Radiofrequency Ablation Making Inroads as Cancer Treatment
 |
 |
RFA: A Talented Treatment Partner |
 |
|
Researchers at the Dana-Farber/Harvard Cancer Center NCI-funded SPORE, working in animal models, have had good success combining RFA with antivascular/antiangiogenesis agents to treat renal cell carcinoma. The approach involves pretreating patients with the agents, reducing blood flow in the tumor and, as a result, heat dissipation. More focused heat means better tumor-cooking ability.
Dr. Wood is involved in a clinical trial that uses an RFA device to deliver a heat-activated drug inside a capsule called a liposome into tumors. The heat generated during ablation causes the intravenous liposome to crack open and deliver its payload: the chemotherapy drug doxorubicin.
|
|
"Those that the knife does not cure are cured by fire," Hippocrates once wrote. This sentiment could also be considered the unofficial mantra of a small cadre of clinicians who have championed a minimally invasive procedure to eradicate small tumors while sparing patients the physical trauma of major surgery.
The technique is radiofrequency ablation (RFA), an outpatient treatment that involves inserting a thin needle, guided by imaging techniques such as ultrasound or computed tomography, through the skin (or, in some cases, through a laparoscopic incision) and into the core of a tumor. At this point, exactly how the tumors are ablated, or "cooked," depends on the device being used. Generally speaking, radiofrequency waves are delivered down the needle shaft to generate just enough heat - slightly over 100° F for 10 to 15 minutes - to destroy the tumor and, hopefully, any cancer cells in the tissue immediately surrounding the tumor, often called the margin.
As a cancer treatment, RFA is moving beyond clinical trials at academic medical centers, with approximately 60,000 procedures performed worldwide last year. Its most extensive use has been to eradicate small liver tumors in patients who aren't candidates for surgery, says Dr. Brad Wood, an interventional radiologist in the Imaging Sciences Program at the NIH Clinical Center. Impressive results with RFA have also been seen in kidney tumors; several other cancers are now feeling RFA's heat.
"Even with surgery and the advances in chemotherapy over the past few years, we still have patients with no other effective options who could be candidates for RF ablation," says Dr. Wood, who also has an appointment in the NCI Surgery Branch.
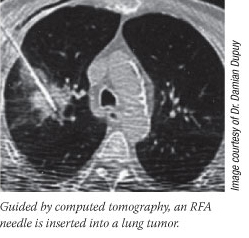 Lung cancer is a therapeutic area for which RFA is under intense study, as these patients commonly have few - if any - treatment options. Lung cancer is a therapeutic area for which RFA is under intense study, as these patients commonly have few - if any - treatment options.
"We see a lot of patients who have early-stage lung cancer who can't have surgery because of emphysema, heart disease, or advanced age," says Dr. Damian Dupuy, a professor of diagnostic imaging at Brown Medical School, who is leading several trials using RFA to treat early-stage lung cancer patients. In data he presented last December on RFA use in 126 inoperable lung cancer patients, more than 50 percent were still alive at 5 years.
Dr. Dupuy and his colleagues also have had promising results in smaller trials combining RFA with radiation, including brachytherapy. Because both RFA and brachytherapy are localized outpatient procedures, the 14-patient trial "was basically a 1-day cancer treatment."
Dr. Dupuy believes that, at least for patients with smaller tumors, RFA eventually could replace surgery in certain situations.
"I think it's the natural progression, especially as technology gets better at detecting cancer early with technologies like proteomics and imaging," he says. "If we can noninvasively stage cancer more accurately, then we can be confident that we're treating a localized disease with a small, localized procedure."
RFA also lends itself nicely to combination treatments. Researchers at the University of Arkansas for Medical Sciences, for example, are testing RFA to support breast lumpectomy, using it to create cancer-free or "negative" margins around the removal site. The approach, explains Dr. V. Suzanne Klimberg, the study's lead investigator, attempts to address a significant problem: As many as 40 percent of the estimated 150,000 women who undergo lumpectomies must have the procedure repeated because the tumor returns, most often at the lumpectomy site.
The available data indicate that clean or negative lumpectomy margins not only have an impact on these local recurrences, she adds, but may also improve survival.
"Margin positivity is really the only prognostic factor that surgeons can affect," Dr. Klimberg says. "Everything else is up to the patient: Is it ER-positive? How big is it? We can't affect that, but we can affect the surgery that we do."
Soon-to-be published data using this RFA-assisted lumpectomy approach in 25 patients report no local recurrences at a median follow-up of 18 months, Dr. Klimberg said. The approach may also eliminate the need for post-lumpectomy radiation therapy, which many women forego because of fear of side effects.
RFA's role in cancer is not strictly curative, Drs. Dupuy and Wood stress. Both are involved in clinical trials using RFA to treat debilitating pain caused by bone metastases.
"I treated a patient yesterday with bone metastases who was in intractable pain and on massive doses of opiates," Dr. Wood recounts. "He … couldn't use his arm and just couldn't function daily. And the morning after the procedure he had no pain, zero."
By Carmen Phillips
|
 |
 |
Prostate Cancer PSA Testing Limitations Demonstrated
A large-scale study of prostate-specific antigen (PSA) screening for prostate cancer concluded that, contrary to current clinical practice, there is no definitive "cutpoint" PSA level to determine the level of risk for the disease, according to an article in the July 6 Journal of the American Medical Association.
The study is based on an analysis of 8,575 healthy men who participated in the placebo arm of the Prostate Cancer Prevention trial; the men in the other study arm received finasteride. Researchers examined the receiver operating characteristic (ROC) curves of various PSA levels, which measures the relative sensitivity (percentage of true disease "positives" detected) and specificity (percentage of true disease "negatives" detected) of the screening method. They found that for detecting any prostate cancer, PSA cutoff values of 1.1, 2.1, 3.1, and 4.1 ng/mL yielded sensitivities of 83.4 percent, 52.6 percent, 32.2 percent, and 20.5 percent, and specificities of 38.9 percent, 72.5 percent, 86.7 percent, and 93.8 percent, respectively.
Study co-author Dr. Howard L. Parnes, chief of NCI's Prostate and Urologic Cancer Research Group, commented, "In the past, many clinicians felt that if the PSA value was below 4, men were essentially free of risk from prostate cancer. That is clearly not the case. Conversely, there was the belief if a man's PSA value was over 4, then a biopsy must be performed. That has also now come into question."
Dr. Parnes added, "It's good to remind people that at every level of PSA, the decision whether to have biopsy needs to be a thoughtful one that takes into account all of a man's risk factors."
High-Risk HPVs Confirmed as Clinical Markers for Cervical Precancer and Cancer
Detection of the two common types of human papillomavirus (HPV) may be useful markers for identifying women who are likely to have or develop cervical cancer and precancerous conditions, according to two related studies published in the July 20 Journal of the National Cancer Institute.
HPV infections cause virtually all cervical cancer. HPV16 infections are implicated in 50 to 60 percent of all women who develop cervical cancer or the precancerous condition cervical intraepithelial neoplasia grade 3 (CIN3). HPV18 is associated with an additional 15 to 20 percent of cervical cancers.
The first study was a randomized, multicenter clinical trial in 5,060 women with mildly abnormal or equivocal Pap smears. Dr. Philip E. Castle of NCI's Division of Cancer Epidemiology and Genetics and colleagues found that women infected with HPV16 were 38 times more likely to develop CIN3 or cervical cancer in the succeeding 2 years than were those with no detectable HPV infection, and 5 times more likely to develop CIN3 or cervical cancer than were women who had other oncogenic HPV types.
In the second study, Dr. Castle and colleagues followed a cohort of 20,512 women for 10 years to see who developed cervical cancer or CIN3. Women with normal Pap smears who were infected with HPV16 or HPV18 at the beginning were 10 times more likely to be diagnosed with cervical cancer or CIN3 than those with any other oncogenic HPV type. Women infected with HPV16 or HPV18 were also more likely to be diagnosed with cervical cancer or CIN3 than women with an abnormal Pap smear.
These results suggest that "identifying HPV16-infected women with normal, equivocal, or mildly abnormal cervical cytology, as well as identifying HPV18-infected women with normal cervical cytology, may be useful for determining those women at high risk of having or developing cervical cancer or CIN3 and therefore their optimal clinical management," said Dr. Castle.
Youth Smoking Behaviors Reduced by State-Sponsored TV Ads
Children aged 10 to 17 smoked less and displayed more favorable antismoking attitudes and beliefs when they had been recently exposed to antismoking TV advertisements sponsored by state public health departments, according to a study published in the July 2005 Archives of Pediatrics and Adolescent Medicine.
The cross-sectional study used nationally representative survey data from 1999-2000. "It is important to maintain a minimal mean exposure of at least one cumulative state-sponsored antitobacco ad per 4-month period for the general teen viewing audience," wrote study lead author Dr. Sherry Emery of the Institute for Health Research and Policy at the University of Illinois at Chicago. The research was funded in part by NCI under the Tobacco Research Initiative on State and Community Interventions.
Survey respondents who had seen at least one such ad within 4 months were less likely to be smokers and more committed to not smoking in the future, the researchers concluded. The study also found that teens were less likely to perceive their friends as smokers and more likely to view smoking as harmful. These results indicate that state-sponsored advertising - while much less frequent than "antitobacco" advertising sponsored by the tobacco industry - is significantly more effective, commented Dr. David Nelson of the Centers for Disease Control and Prevention.
Study investigators used Nielsen commercial ratings to determine broadcast and cable TV audience exposure to the antitobacco ads. This information was merged with data from the University of Michigan's Monitoring the Future study, which measured student characteristics, smoking-related attitudes and beliefs, and self-reported tobacco use by 8th, 10th, and 12th graders.
New Clues in Regulation of DNA Duplication
When a cell divides, it generates an identical copy of the three billion letters of the nuclear genome - or at least it tries to. Sometimes mistakes occur, which can lead to cancer. Researchers are now one step closer to understanding how the DNA duplication process works, according to a report in the July 10 online edition of Nature Cell Biology.
"It's a significant result that will allow us to rethink strategies for designing drugs" that prevent errors in DNA duplication, said Dr. Philipp Kaldis of the Mouse Cancer Genetics Program in NCI's Center for Cancer Research.
Much of the cell-division process is regulated by a group of proteins called cyclin-dependent kinases. The proteins have drawn much attention from basic biologists because mutations in them can trigger cancer.
Dr. Kaldis and his colleagues made genetically modified mice that lacked a particular cyclin-dependent kinase. They expected the mice to develop rampant tumors and were surprised when they did not. "It turns out there are two parallel pathways and [more severe cancers] occur only if both are inhibited," said Dr. Kaldis. He added that scientists have been trying to prove a role of a cellular protein called mammalian Cdc2 in DNA duplication for 20 years. "We can change the textbooks now," he said.
|
 |
 |

Following are newly released NCI research funding opportunities:
 |
 |
Featured Meetings and Events |
 |
|
Etiology, Prevention, and Treatment of Hepatocellular Carcinoma (R01 and R21)
PA-05-137
Application Receipt Dates: Oct. 1, 2005; Feb. 1, June 1, and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1 and June 1, 2008
This funding opportunity will use the R01 and R21 award mechanisms. For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa
_id=2846.
Inquiries: Dr. John Cole - jc121b@nih.gov; Dr. Asad Umar - au17z@mail.nih.gov; Dr. Heng Xie - XieHe@mail.nih.gov
Etiology, Prevention, and Treatment of Hepatocellular Carcinoma (P01)
PA-05-138
Application Receipt Dates: Oct. 1, 2005; Feb. 1, June 1, and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1 and June 1, 2008
This funding opportunity will use the P01 award mechanism.
For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2847.
Inquiries: Dr. John Cole - jc121b@nih.gov; Dr. Asad Umar - au17z@mail.nih.gov; Dr. Heng Xie - XieHe@mail.nih.gov
Short-Term Courses in Human Embryonic Stem Cell Culture Techniques
PAR-05-133
Letter of Intent Receipt Date: August 8, 2005.
Application Receipt Date: September 8, 2005
This is a renewal of PA-02-054. This funding opportunity will use the T15 award mechanism.
For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2848.
Inquiries: Dr. John Sogn - JS150x@nih.gov
Ruth L. Kirschstein NRSA Fellowships in Cancer Nanotechnology Research
RFA-CA-06-010
Application Receipt Date: November 16, 2005
This funding opportunity will use the F32 and F33 award mechanisms.
For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2849.
Inquiries: Dr. Gregory J. Downing - downingg@mail.nih.gov
Research on Research Integrity
RFA-NR-06-001
Letter of Intent Receipt Date: August 16, 2005.
Application Receipt Date: September 16, 2005
This is a renewal of RFA-NS-05-003. This funding opportunity will use the R01 award mechanism.
For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2850.
Inquiries: Dr. Ann O'Mara - Omaraa@mail.nih.gov.
For comprehensive information about NCI funding priorities and opportunities, go to http://www.cancer.gov/researchandfunding.
The NIH Roadmap for Medical Research Funding provides a framework of the priorities NIH must address to optimize its research portfolio. It identifies the most compelling opportunities in three main areas: new pathways to discovery, research teams of the future, and re-engineering the clinical research enterprise. For information on additional Roadmap funding opportunities, go to http://nihroadmap.nih.gov.
|
 |
 |

Targeted Combination Therapy for Advanced Solid Tumors
Name of the Trial
Phase I Randomized Study of Sorafenib and Bevacizumab in Patients with Refractory, Metastatic, or Unresectable Solid Tumors (NCI-05-C-0022). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-05-C-0022.
 Principal Investigator
Principal Investigator
Dr. Elise Kohn, NCI Center for Cancer Research
Why Is This Trial Important?
Advanced-stage solid tumors are generally difficult to treat with established forms of therapy. The prognosis for patients with advanced-stage solid tumors is often relatively poor, not only because their tumors are frequently not amenable to standard treatments but also because their cancer has likely spread (metastasized) to other parts of the body.
Solid tumors depend upon new blood vessel formation - a process known as angiogenesis - to obtain oxygen and nutrients for continued growth. A variety of antiangiogenic drugs targeting this "Achilles' heel" has been under development for several years.
An angiogenesis inhibitor called bevacizumab (Avastin) received FDA approval in 2004 for the treatment of advanced colorectal cancer. Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF), a protein produced by many types of cancer cells that stimulates new blood vessel growth in tumors.
In this study, researchers are assessing the safety and combined effectiveness of bevacizumab and a second drug called sorafenib. Sorafenib also inhibits angiogenesis, but it does so by blocking the activity of proteins that are activated by VEGF (namely, VEGF receptor proteins). Importantly, the antitumor effects of sorafenib extend beyond VEGF receptor protein inhibition to include inhibition of other proteins that may be involved in cancer cell proliferation and tumor growth.
"We hope that the antitumor effects of these two targeted agents will prove mutually reinforcing when given in combination," said Dr. Kohn.
Who Can Join This Trial?
Researchers plan to enroll up to 38 patients aged 18 and older who have been diagnosed with a solid-tumor malignancy. See the complete list of eligibility criteria at http://cancer.gov/clinicaltrials/NCI-05-C-0022.
Where Is This Trial Taking Place?
The trial is taking place at the NIH Clinical Center in Bethesda, Maryland.
Contact Information
For more information about this and other intramural trials, contact the NCI Clinical Studies Support Center at 1-888-NCI-1937. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |
 |
 |
CCR Grand Rounds |
 |
|
July 26: Dr. David H. Johnson, Cornelius A. Craig Professor of Medicine, Director, Division of Hematology/Oncology, Deputy Director, Vanderbilt-Ingram Cancer Center "Targeting Inflammation in Non-Small-Cell Lung Cancer"
CCR Grand Rounds are held 8:30 to 9:30 a.m. at the NIH campus in Bethesda, Md., in the Clinical Center's Lipsett Amphitheater.
Please Note: CCR Grand Rounds will not be held during the month of August. They will resume on September 13.
|
|
2005 NIH Director's Awards Ceremony
On July 14, several NCI employees were honored at the 2005 NIH Director's Awards Ceremony, which was held on the NIH campus in Bethesda, Md. NIH Director Dr. Elias Zerhouni presided over the ceremony and the presentation of more than 60 awards to NIH employees. "By no means are the colleagues we're honoring today the only ones who have contributed significantly to our mission," Dr. Zerhouni said. "I really appreciate the professionalism, dedication, and perseverance of the entire NIH staff. But it's really a pleasure to take some time to honor some of our colleagues."
The following NCI employees received awards:
- NIH Director's Award for special efforts beyond regular duty to NIH: Drs. Malcolm Smith, Richard Simon, Alan Hildesheim, and J. Carl Barrett
- NIH Mentoring Award: Dr. Peter Blumberg
- NIH Director's Group Award: Jacquelyn Goldberg, Jeanne Adler, and Dr. Barry Anderson - Pediatric Clinical Investigations and Research Branch Team; Christina Bruce and Viola Black - EEO Restructuring Team; James Dickens - Roadmap Central Implementation Team
- PHS NIH Commissioned Corps Award for cancer-related health disparities research: Capt. Linda Brown.
Cancer PSAs Air on Soap Opera
Soap opera viewers know a bit more about cancer thanks to the Hollywood, Health & Society (HH&S) program, funded by NCI and the Centers for Disease Control and Prevention.
Producers for CBS-TV's As the World Turns developed two story lines with scientific feedback from NCI - one involving a main character with breast cancer and another involving a pregnant teen who smokes. The ongoing story lines were bracketed by public service announcements (PSAs) featuring stars from the show. A mammography PSA aired on June 23 and teen smoking and pregnancy PSAs aired on July 11 and 13.
The Cancer Information Service reported an increase in calls related to the two topics, with many callers citing the show as the source of their curiosity. HH&S is conducting research to determine how many viewers feel they know more about the health issues mentioned after watching episodes with the cancer-related story lines.
Based at the University of Southern California's Annenberg School for Communication, HH&S is billed as a "one-stop shop to provide entertainment industry professionals with accurate and timely information for health story lines."
Blumenthal to Head New CCR Nanobiology Program
Dr. Robert Blumenthal has been appointed as director of the new Center for Cancer Research Nanobiology Program (CCRNP), which combines physics, computational sciences, and biology to develop nanoscale material-based strategies for the prevention, diagnosis, and treatment of cancer. The program, previously the Laboratory of Experimental and Computational Biology, will interface with NCI's main Nanotechnology Program, other CCR basic research labs and clinical branches, and the extramural community.
|
|
 |
|