Director's Message
Research Areas and Partnering Nations Featured in this Portfolio
Introduction
Understanding the Causes and Mechanisms of Cancer
Accelerating Progress in Cancer Prevention
Improving Early Detection and Diagnosis
Developing Effective and Efficient Treatments
Understanding the Factors that Influence Cancer Outcomes
Improving the Quality of Cancer Care
Improving the Quality of Life for Cancer Patients, Survivors, and Their Families
Improving Cancer Communications
Scientist Exchanges and Training Programs
Building the Capacity and Infrastructure for Cancer Research and Care
Director's Message
The National Cancer Institute (NCI) plays an important role in promoting global health and
contributing to the economy and security of nations around the world. As this portfolio
demonstrates, NCI's international activities are both broad and deep. However, the
activities described here are only a small sample of the Institute's efforts.
NCI is committed to playing an even greater role in international cancer control in the future.
That is evidenced by our involvement in emerging international collaborations, most notably the
World Health Organization's (WHO) global cancer prevention and control resolution.
During the 2005 World Health Assembly, WHO passed Resolution WHA58.22, a first-of-its-kind
resolution calling for improved cancer prevention measures, improved early detection and treatment,
and more palliative care in all WHO Member State countries.
NCI scientists have joined some of the world's leading cancer control researchers in providing WHO
with scientific expertise to develop and implement this global strategy. Mark Clanton, M.D., M.P.H.,
Deputy Director, NCI, and Deputy Director for Cancer Care and Delivery Systems, serves as the
Institute's representative to the WHO Director-General's Cancer Advisory Committee and WHO's
Cancer Technical Working Group.
Dr. Clanton is also the NCI lead for another important project with the International Atomic Energy
Agency (IAEA) as part of a program called the Program of Action for Cancer Therapy, or PACT.
IAEA has provided radiation therapy machines in low-resource settings for the last decade, giving
support to treatment centers in developing countries so they can deliver appropriate radiation
therapy to patients. IAEA is now greatly expanding these cancer control activities through the
launch of the PACT Alliance - an alliance of cancer organizations from across the globe to help
develop and implement cancer control programs in developing countries.
NCI will help support a pilot of this expanded PACT program, including bringing together a team of
experts in cancer control from the United States to assist in its development and implementation.
It is an inspiration to witness the effort put forth by scientists and health care providers around the
world to improve the health of all humans, regardless of race, gender, age, or religion. I am proud -
as I believe the entire U.S. cancer community should be - of NCI's continued commitment to
reducing the global cancer burden.
John E. Niederhuber, M.D.
Director, National Cancer Institute
Back to Top
Research Areas and Partnering Nations Featured in this Portfolio
Afghanistan
Argentina
Australia - Melbourne - Perth - Queensland
Austria
Bangladesh
Belarus
Belgium - Brussels
Bolivia
Brazil - Salvador - Rio de Janeiro
Cameroon
Canada - Alberta - Toronto - Vancouver
- Winnipeg
Chile
China - Beijing - Chongqing - Guangxi
- Guangzhou - Hong Kong - Lixian - Qingdao
- Shanghai
Costa Rica
Croatia
Cyprus
Czech Republic
Egypt - Cairo
England - Birmingham - London
Estonia
France
Germany - Göttingen - Heidelberg - Würzburg
Ghana
Greece
Hungary
India - Chennai
Iran
Iraq
Ireland - Cork - Dublin - Galway
Israel - Rehovot
Italy
|
Japan - Tokyo
Jordan - Amman
Kenya
Lithuania
Madagascar
Malaysia
Mexico - Mexico City - Sonora
Netherlands - Rotterdam
Northern Ireland - Belfast
Norway - Oslo
Pakistan
Palestinian Authority
Peru
Poland
Portugal - Lisbon
Romania
Russia - Moscow
Senegal - Dakar
Singapore
Slovakia
South Africa - Cape Town
Spain - Barcelona
Sweden - Stockholm
Switzerland
Tanzania
Thailand
Turkey
Uganda - Kampala
Ukraine - Chernobyl
United Arab Emirates
Uruguay
Venezuela
Zambia |
Back to Top
Introduction
The global burden of cancer is large and growing larger. Each year, more than 11 million
people are diagnosed with cancer worldwide. By the year 2020, this number is expected
to increase to 16 million. In addition, cancer causes more than 8 million deaths each year
-- or approximately 13 percent of all deaths worldwide.
In many developed countries, including the United States, cancer accounts for more than
20 percent of all deaths. In less developed countries, overall cancer rates are generally
lower and cancer accounts for a lower percentage of deaths. However, it is within
developing countries that cancer is projected to increase most rapidly over the next
few decades. Unless current trends change, cancer in developing countries is expected
to represent 70 percent of the global cancer burden by the year 2030, a statistic driven
by demographic shifts toward more elderly populations and the movement toward more
Western lifestyles, most notably increased per capita tobacco consumption and higher
fat-lower fiber diets.
In the National Cancer Act of 1971, the National Cancer Institute (NCI) was charged to:
"Collect, analyze, and disseminate all data useful in the prevention, diagnosis, and treatment
of cancer…[and to] disseminate insofar as feasible the results of cancer research
undertaken in any country for the use of any person involved in cancer research in any
country." In addition, the Institute was directed to: "Support research in the cancer field
outside the United States by highly qualified foreign nationals…; support collaborative
research involving American and foreign participants; and support the training of
American scientists abroad and foreign scientists in the United States."
Clearly, it was the intent of the U.S. Congress that NCI should not only address the
challenge of cancer among American citizens but also among the citizens of all nations.
This challenge is daunting indeed, but it is one from which we cannot shrink.
A global perspective offers a myriad of research opportunities that a U.S.-only research
focus would not afford. For example, international studies enable us to investigate "rare"
cancers -- such as certain inherited, familial types of kidney cancer, melanoma, and other
cancers -- by providing access to much larger populations of patients than can be found
within the confines of our national borders. A global perspective also opens to us the
diversity of environments occupied by humans, providing unique opportunities to explore
relationships between genes and specific environmental exposures, including infectious
agents that may be associated with cancer.
Furthermore, international programs give us access to resources found only in other
countries. These resources allow for consistency in diagnosis and tumor classification.
NCI also recognizes the importance of investing in developing countries, especially with
respect to improving research and health care infrastructure. No nation exists in a
vacuum and cancer does not recognize international borders. NCI is committed to sharing
our expertise to foster cancer research and build research and health care infrastructure
around the world. Just as American researchers benefit from a broader perspective by
engaging in research outside U.S. borders, international researchers make significant
contributions to NCI's overall mission while acquiring knowledge, skills, and abilities to
enhance the research environment in their home countries.
The mission to train both American and foreign scientists to battle cancer is one that
NCI takes seriously. When we cooperate internationally to address a shared health
burden, knowledge is expanded, solutions are discovered more efficiently, and the health
of all people is improved.
Monitoring NCI's international activities, many of which are managed within the
Institute's intramural and extramural divisions, is the responsibility of the Office of
International Affairs (OIA). OIA also directs a range of activities that are intended to
catalyze research advances through individual and group training and through fostering
interactions between cancer researchers in the United States and abroad. These latter
activities include initiating, developing, and implementing bilateral and multilateral
agreements to share information and expertise with other nations and groups of nations.
One excellent example of this type of OIA activity is the Middle East Cancer Consortium
(MECC), which is described in
Building the Capacity and Infrastructure for Cancer Research and Care.
OIA also coordinates NCI's involvement in the global clinical trials enterprise. This key role for
OIA is demonstrated by the work of NCI's Liaison Office in Brussels, Belgium (see The NCI Liaison Office in Europe).
This report provides an overview and brief descriptions of NCI's international cancer control
and research programs, as well as the Institute's efforts to share scientific knowledge, build
and support cancer research infrastructure in other nations, and improve the delivery of
cancer information and care to people around the globe. You will find compelling reports that
demonstrate NCI's efforts towards addressing the global challenge of cancer. However, keep in
mind that the efforts and activities presented here are just a sample of the work being done by
NCI scientists, our grantees, and our international partners.
Back to Top
Understanding the Causes and Mechanisms of Cancer
Cancer is invariably caused by changes in the function of genes that regulate vital cellular processes, such as
growth and proliferation and programmed cell death (apoptosis). These changes may be caused by mutations in the
DNA sequence of a person's genes or by epigenetic events, in which gene expression is altered without the
occurrence of DNA mutations.
NCI and its partners worldwide are striving to identify the genetic, epigenetic, and environmental factors that
contribute to the development of cancer. We are also seeking to understand how genes and the environment interact
to influence the cancer process. In this effort, we are developing innovative technologies and harnessing the power
of bioinformatics.
Identifying and characterizing the environmental causes of cancer are crucial if we are to
develop effective strategies for cancer prevention and control. Two major NCI-supported
efforts in this area are the International Agency for Research on Cancer's (IARC) Program
on the Evaluation of Carcinogenic Risks to Humans (see below) and the Institute's
long-standing partnership with Chinese health authorities to investigate the effects of
occupational exposure to benzene, which is described in Benzene and Cancer: East Meets West.
Other NCI-supported international activities that focus on environmental risk factors for
cancer include the following:
NCI's Chernobyl Research Unit (CRU) is participating in three epidemiologic studies related
to the 1986 accident at the Chernobyl nuclear facility in Ukraine: 1) a study of leukemia,
lymphoma, and other blood diseases among Ukrainian clean-up workers; 2) a study of
thyroid cancer and other thyroid diseases among Ukrainians who were exposed as children
to radiation from the Chernobyl reactor; and 3) a study of thyroid cancer and thyroid
diseases among Belarusians who were exposed as children to radiation from Chernobyl.
The Chernobyl facility is located about 12 kilometers south of the Ukraine-Belarus border.
The thyroid studies involve biannual thyroid gland examinations of approximately
12,000 to 13,000 individuals in each country who were exposed to radiation from the
accident when they were 0 to 18 years of age and who had thyroid radiation dose
measurements made in the weeks following the accident.
This program has relevance to international concerns about nuclear terrorism because
the Chernobyl exposures are similar to those expected from a "dirty bomb" attack.
Arsenic exposure is an established risk factor for kidney, bladder, lung, and skin cancers,
but the relationship between arsenic, its metabolites, and gastrointestinal cancer has
received only limited attention. In this study, researchers are investigating the role of
arsenic in the development of gastrointestinal cancer in two geographic areas of Sonora,
Mexico that have different levels of arsenic in their drinking water supplies. Researchers
from the University of Arizona, the University of Sonora, and the Technological Institute
of Sonora - who are experienced in arsenic research, exposure assessment, and cancer
research - are conducting the study.
Historically, attempts to link environmental exposures to cancer have frequently relied
on population surveys, which provide indirect information in the form of responses to
questionnaires or personal interviews. The inherent errors associated with these kinds of
assessments have long hindered the identification and quantification of important exposures.
Rather than relying on these traditional methods of dose estimation and extrapolation
from high-dose exposures in rodent models, researchers at the University of Alberta
are developing new technology to provide sufficiently sensitive DNA damage measurements
that would permit more realistic assessments of environmental risk. The technology
would also be useful for measuring the DNA damage induced by anticancer agents and
for studying DNA repair, which is an essential cellular defense mechanism against DNA
damage and cancer development.
Although our understanding of the causes and mechanisms of cancer initiation and progression
has expanded rapidly, much remains to be learned. We now recognize that a person's
susceptibility to cancer can be governed by the interaction of genetic and environmental
factors. If we are to continue making progress in the prevention and treatment of cancer,
it is clear we must increase our understanding of the interplay between susceptibility genes
and the environment. To achieve our goal of eliminating the suffering and death due to
cancer, broad collaboration of persons, organizations, and nations in this effort is crucial.
A prime example of such collaboration is the InterLymph Consortium, more formally
known as the International Consortium of Investigators Working on Non-Hodgkin's
Lymphoma Epidemiologic Studies. This consortium is described
in InterLymph Leads Global Research in Non-Hodgkin Lymphoma. Other
examples include the following:
More than 15 years ago, researchers from NCI and the Chinese Center for Disease Control (CCDC), which was then
called the Chinese Academy of Preventive Medicine, formed a partnership to evaluate the cancer risks associated
with occupational exposure to benzene. This collaboration grew out of a Chinese national health survey that showed
that benzene exposure was common in Chinese industry and that exposed workers had increased risks for leukemia
and other diseases. In view of these findings, NCI and Chinese scientists decided to characterize the spectrum of
diseases caused by benzene, to relate benzene exposure levels to disease risks, and to define the mechanisms of
benzene carcinogenicity in humans.
This partnership, one of the longest ever undertaken between American and Chinese scientists, engages NCI experts
in cancer epidemiology, industrial exposure assessment, and the pathology of blood-system diseases. This is a significant
Chinese public health problem that also has important implications for the United States and other countries. To
date, findings from this collaboration have contributed to lowering the occupational standard for benzene exposure in
China and have greatly affected the risk assessment process for environmental benzene exposure in the United States.
The initial study included 75,000 benzene-exposed workers and 35,000 workers without occupational benzene exposure
who were employed between 1972 and 1987 at more than 700 factories in 12 cities throughout China. Data on work
history and exposure were collected, along with follow-up data on each worker to ascertain vital status, cause of
death for deceased workers, and incidence of any hematopoietic or lymphoproliferative malignancy or related disorder.
This study produced three key findings that were reported in a landmark paper published in 1997.1 First, significantly
increased risks were found among exposed workers for developing acute non-lymphocytic leukemia (ANLL) and
related myelodysplastic syndromes (MDS) and for non-Hodgkin lymphoma (NHL). Second, elevated risks for the
combined grouping of ANLL/MDS and for NHL occurred at average exposures of less than 10 parts-per-million (ppm).
Third, the developmental pattern of disease differed between ANLL/MDS and NHL, i.e., the former diseases were
linked to more recent benzene exposure (less than 10 years before diagnosis), whereas NHL was more related to
exposures that occurred 10 or more years earlier).
Subsequent studies revealed the presence of leukemia-associated chromosomal aberrations in benzene-exposed
workers and showed that susceptibility to the adverse health effects of benzene can vary on the basis of an individual's
genetic make up. One study reported in 2004 demonstrated that the toxic effects of benzene on mature and early
progenitor blood cells can occur at air levels of 1 ppm or less and suggested that these effects may be particularly
evident among genetically susceptible individuals.2 These latter findings have relevance for American workers
because the current occupational standard for benzene exposure, established by the U.S. Occupational Safety and
Health Administration (OSHA), is 1 ppm during a 40-hour work week.
1 Hayes R, Yin S-N, Dosemeci M, Li G-L, Wacholder S, Travis L, Li C-Y, Rothman N, Hoover R, Linet M. Benzene and the dose-related incidence of hematologic
neoplasms in China. Journal of the National Cancer Institute, July 16, 1997; 89(14):1065-1071.
2 Lan Q, Zhang L, Li G, Vermeulen R, Weinberg R, Dosemeci M, Rappaport S, Shen M, Alter B, Wu Y, Kopp W, Waidyanatha S, Rabkin C, Guo W, Chanock S, Hayes R,
Linet M, Kim S, Yin S, Rothman N, Smith M. Hematotoxicity in workers exposed to low levels of benzene. Science, December 3, 2004; 306(5702):1774-1776.
|
In the year 2000, an estimated half-million cases of head and neck cancer were diagnosed
worldwide and 300,000 people died of the disease. Most of these cancers can be attributed
to tobacco and alcohol use, but other risk factors that may play a role include
viral infection, occupational exposures, radiation exposure, dietary factors, and genetic
susceptibility. In 2004, NCI and the World Health Organization (WHO) joined forces to
establish the INHANCE Consortium, which is comprised of research groups that are
conducting large molecular epidemiology studies of head and neck cancer. These studies
should increase our understanding of the causes and mechanisms of head and neck cancer
worldwide. The consortium is managed by IARC and includes investigators from the
United States, France, the Czech Republic, Slovakia, Romania, Hungary, Poland, Russia,
Spain, Costa Rica, Italy, Switzerland, Brazil, Argentina, Germany, the United Kingdom,
Norway, Greece, Estonia, and Croatia.
NCI scientists and researchers from China's Institute for Viral Disease Control and
Prevention, Beijing and the Wuzhou Red Cross Hospital, Guangxi Province are attempting
to characterize genes associated with susceptibility or resistance to the development
of nasopharyngeal carcinoma (NPC). The population in Guangxi Province has a high
incidence of NPC. This population offers a unique model of this human malignancy for
understanding a multistep carcinogenic process that involves a virus (EBV), environmental
carcinogens (dietary and other causes), and genetic factors.
Similarly, NCI scientists have joined forces with the Department of Infectious Diseases at
Peking University First Hospital, Beijing to study how outcomes of HBV exposure and
infection are influenced by host genetic factors in the Chinese population. In China, more
than 120 million individuals are infected with HBV. Among persons persistently infected
with this virus, 10 to 30 percent will develop cirrhosis and liver cancer.
In South Africa, squamous cell esophageal cancer occurs with high frequency, causing
the majority of cancer-related deaths among black males. Better understanding of the
molecular events leading to the development of this cancer will allow earlier diagnosis,
the development of better therapeutic strategies, and enhanced prevention of metastasis.
Reduced expression of NDRG1 (N-Myc Downstream Regulated Gene 1) - which has
been implicated in cell differentiation, cell proliferation, and cancer cell metastasis -
has been found in poorly differentiated esophageal tumors.
Despite the knowledge that prostate cancer occurs with high frequency in men of African
descent in the Americas, little information is available regarding the epidemiology of
prostate cancer in native African men, even though prostate cancer seems to be prevalent
in that population as well. The objective of this study is to examine the role of genes
that regulate the physiological disposition of testosterone in the development of prostate
cancer and to evaluate whether these genes explain, in part, ethnic differences in prostate
cancer rates. An understanding of the complex interplay of genetic variability at multiple
loci and of environmental agents may improve our understanding of ethnic differences in
prostate cancer development and risk prediction.
caused by tobacco and alcohol use, only a minority of heavy smokers and heavy drinkers
will develop these cancers. A possible explanation for this phenomenon is that individuals
vary widely in their metabolism of carcinogenic products, internal dose levels of these
products and/or their metabolites, DNA repair capacity, and cell-cycle control mechanisms
due to genetic factors. In separate investigations, the researchers will study the role
of 45 genes that may be involved in the susceptibility to lung and UADT cancers.
The goal of this study is to identify single-nucleotide polymorphisms (SNPs) and haplotypes
in steroid hormone metabolizing genes, genes in the insulin-like growth factor (IGF)
pathway, and genes that encode related receptor proteins. SNPs are DNA sequence
variations that arise from single nucleotide (A, T, C, or G) changes. Haplotypes are sets
of genes that are linked closely enough to be inherited as a unit.

| |
Woman smoking
|
In the study, the investigators will have access to prospectively
gathered plasma samples, genetic material, anthropometric measurements,
and extensive questionnaire data on diet, physical activity,
exogenous hormone use, smoking, and other lifestyle factors
from over 790,000 men and women worldwide. The biological
samples and other data are from the following large prospective
cohorts: the American Cancer Society's Cancer Prevention Study II;
Harvard University's Harvard Cohort Studies; IARC's European
Prospective Investigation into Cancer and Nutrition (EPIC)
Study; the University of Hawaii and the University of Southern
California's Multiethnic Cohort Study; and two NCI intramural
cohorts (the Prostate, Lung, Colon, and Ovarian Cancer Screening
Study and the Alpha-Tocopherol, Beta-Carotene Prevention Trial).
The ultimate goal of this study is to provide the foundation for
reducing the public health burden of breast and prostate cancers.
Over the past few decades, the incidence of cutaneous melanoma has increased dramatically
in light-skinned populations worldwide. The Australian state of Queensland has the
highest incidence of cutaneous melanoma in the world, with lifetime incidences of 1 in 13
males and 1 in 16 females. Although these rates are almost five times greater than those
in the United States, the shapes of the age-specific incidence curves are almost the same in
the two populations, suggesting similar causal factors.
In this study, the researchers will extend several earlier large-scale investigations into
the molecular genetics and genetic epidemiology of melanoma and its risk factors (in
particular, nevus density and pigmentation). They will analyze DNA specimens obtained
from 6,248 individuals. These analyses will include DNA sequencing and singlenucleotide
polymorphism (SNP) analyses of genes in the cell-cycle control and pigmentation
pathways. The researchers will look for associations of melanoma risk variables
with SNPs and environmental risk factors. They will also investigate whether melanoma
in childhood or adolescence can be explained solely by the same risk factors that operate
in adults or whether affected children or adolescents carry rare alleles in cell cycle or
pigmentation genes.
|
The InterLymph Consortium - more formally known as the International Consortium of Investigators Working on
Non-Hodgkin Lymphoma Epidemiologic Studies - is an open scientific forum for epidemiologic research that was
formed in 2001. The Consortium is comprised of international investigators who have completed or have ongoing
case-control studies of non-Hodgkin lymphoma and who discuss and undertake research projects that pool data
across studies or otherwise conduct collaborative research. The ultimate goal of InterLymph is to speed progress
toward understanding the etiology of non-Hodgkin lymphomas.
Support for the logistical needs of InterLymph is provided by NCI, the International Agency for Research on Cancer
(IARC), and the United Kingdom's Leukaemia Research Fund. Some InterLymph investigators also receive grant
support for research projects from NCI. Investigators from the United States, Europe, Canada, Australia, the Middle
East, and Asia have participated in the Consortium.
Eight InterLymph working groups develop ideas for coordinated research in the areas of diet and behavior, family
studies, genotyping, immunology, infections, occupation, pathology, and sunlight.
Recently reported studies investigated the relationships between cigarette smoking, alcohol consumption, and common
genetic variants in immune system and inflammatory response genes and the risk of non-Hodgkin lymphoma.1,2,3
 |
Interlymph 5th Annual Meeting
Washington, D.C.
March 30-April 1, 2006 |
1 Morton L, Hartge P, Holford T, Holly E, Chiu B, Vineis P, Stagnaro E, Willett E, Franceschi S, La Vecchia C, Hughes
A, Cozen W, Davis S, Severson R, Bernstein L, Mayne S, Dee F, Cerhan J, Zheng T. Cigarette smoking and risk of
non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph).
Cancer Epidemiology, Biomarkers & Prevention, April 2005; 14(4):925-933.
2 Morton L, Zheng T, Holford T, Holly E, Chiu B, Costantini A, Stagnaro E, Willett E, Dal Maso L, Serraino D, Chang E,
Cozen W, Davis S, Severson R, Bernstein L, Mayne S, Dee F, Cerhan J, Hartge P; InterLymph Consortium. Alcohol
consumption and risk of non-Hodgkin lymphoma: a pooled analysis. Lancet Oncology, July 2005; 6(7):469-476.
3 Rothman N, Skibola C, Wang S, Morgan G, Lan Q, Smith M, Spinelli J, Willett E, De Sanjose S, Cocco P, Berndt S,
Brennan P, Brooks-Wilson A, Wacholder S, Becker N, Hartge P, Zheng T, Roman E, Holly E, Boffetta P, Armstrong B,
Cozen W, Linet M, Bosch F, Ennas M, Holford T, Gallagher R, Rollinson S, Bracci P, Cerhan J, Whitby D, Moore P,
Leaderer B, Lai A, Spink C, Davis S, Bosch R, Scarpa A, Zhang Y, Severson R, Yeager M, Chanock S, Nieters A.
Genetic variation in TNF and IL10 and risk of non-Hodgkin
lymphoma: a report from the InterLymph Consortium. Lancet Oncology, January 2006; 7(1):27-38.
|
CLL is the most common form of leukemia among adults in the Western world. No
specific environmental risk factors have been established for CLL, but epidemiologic and
family studies indicate that 8 to 10 percent of CLL cases involve inherited susceptibility
to the disease. Because it was recognized that no single institution could recruit a sufficient
number of CLL families to achieve the goal of identifying a susceptibility gene(s),
the International Familial CLL Consortium was established in 2002. Enrolling new CLL
families and coordinating research efforts among the participating centers are core tasks
of the consortium. Countries participating in the consortium include the United States,
the United Kingdom, France, and Italy.
These large cancer family registries are resources for population-based, case-control
family studies conducted by the Centre for Molecular, Environmental, Genetic and
Analytic Epidemiology at the University of Melbourne's School of Population Health.
The Australian Breast Cancer Family Registry is also part of the Cooperative Family
Registry for Breast Cancer Studies (CFRBCS), which is an international collaboration
involving six registries that was initiated by NCI in 1995 to provide the scientific community
with a resource for interdisciplinary and translational breast cancer research. CFRBCS
resources include a repository of biological specimens from a racially and ethnically
diverse set of families that have a history of breast cancer and a large, computerized database
containing both genetic and environmental risk information.
Similarly, the Australasian Colorectal Cancer Family Registry is part of the Cooperative
Family Registry for Colorectal Cancer Studies (CFRCCS), which is another international
collaboration initiated by NCI in 1998. The six CFRCCS registries perform several tasks,
including the assembly and maintenance of comprehensive lists of families with histories
of colorectal cancer, the collection of detailed information about possible factors involved
in the cancer process, and the storage of blood samples and tumor biopsy specimens from
family members for research purposes. The data and samples collected by the participating
registries are available to researchers worldwide.
These registries collect and store personal and family health information from residents
of Ontario, Canada, who have a family history of breast or colorectal cancer and who
are willing to participate in research studies. The registries provide an infrastructure for
current and future research on breast and colorectal cancer genetics and new preventive
and therapeutic strategies to combat these diseases. The Ontario registries are partner registries
in the Cooperative Family Registry for Breast Cancer Studies and the Cooperative
Family Registry for Colorectal Cancer Studies (see
The International Familial Chronic Lymphocytic Leukemia (CLL) Consortium to identify
inherited susceptibility genes).
The study of kidney cancers associated with rare genetic disorders is limited by the infrequent
occurrence of the individual diseases and the small numbers of patients available
for study in any one nation. Therefore, international collaboration is essential.
One such collaboration between NCI scientists and investigators at the University of
Manitoba in Winnipeg, Canada and the University of Birmingham in the United Kingdom
led to the successful cloning of the Birt Hogg Dube (BHD) gene, which is associated with
a rare hereditary syndrome characterized, in part, by a high predisposition to malignant
kidney tumors that are often bilateral and multifocal.
Ongoing efforts include the identification of additional patients with familial kidney
cancers of undetermined etiology worldwide to increase our ability to identify major
genes that contribute to the development of kidney tumors.
Although HPV infection is an established cause of cervical cancer, it is not known
whether viral load influences the progression from localized cervical carcinoma in situ
(CIS) to invasive cancer and/or interacts with host genetic factors. Since clinical intervention
precludes direct observation of this progression, unconventional approaches are
required. The investigators are attempting to: 1) quantify the absolute and relative risks
for CIS and invasive cancer as a function of time since the detection of HPV and high
viral load of HPV strain 16 (HPV-16); 2) assess whether a persistent HPV-16 high viral
load is a determinant of CIS and invasive cancer development; 3) assess whether a
specific histocompatibility antigen genotype is associated with risks for CIS and invasive
cancer and if the association is mediated via a higher viral load and/or persistence of HPV infection; and 4) assess whether infection with the bacterium Chlamydia trachomatis
is associated with risks for CIS and invasive cancer. The researchers will take advantage
of Sweden's extensive documentation in computerized registries of its population-based
Pap smear screening program, its ascertainment of all incident cases of cervical CIS and
invasive cancer, and archived Pap smears and tissue specimens.
 | 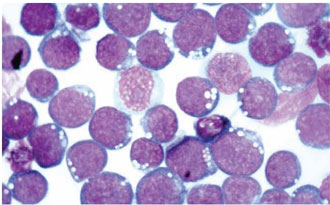 |
| Molecular
Diagnosis of Burkitt's
Lymphoma | Epstein-Barr
Virus (EBV) |
EBV is ubiquitous worldwide, with more than 80 percent of people over the age of 30
having been infected. Once EBV infection has occurred, it persists for the lifetime of the
individual. EBV infection is strongly associated with the development of several cancers,
including Hodgkin disease and Burkitt lymphoma (BL). Three variants of BL have been
identified: endemic, sporadic, and human immunodeficiency virus-1 (HIV-1)-associated.
However, the molecular differences between these variants have not been well characterized.
BL is also commonly identified in patients with acquired immunodeficiency
syndrome (AIDS), with the EBV association being more common in developing countries.
In this study, scientists from the University of Miami; the University of North Carolina,
Chapel Hill; the Federal University of Bahia in Salvador, Brazil; and the Brazilian Pediatric
Non-Hodgkin Lymphoma Treatment Group are molecularly characterizing primary
EBV-positive BLs using virus-specific microarrays. Other goals of the study are to identify
the mechanism by which the common antiretroviral drug azidothymidine (AZT) induces
apoptosis (cell suicide) in EBV-positive BL and to use the data to develop novel therapies.
The study is supported by independent grants and by supplemental funding from the
NCI-sponsored AIDS-Associated Malignancies Clinical Trials Consortium.
|
Since 1972, the International Agency for Research on Cancer (IARC), which is part of the World Health Organization,
has published a series of monographs on the carcinogenic risks posed to humans by a variety of agents, mixtures,
exposures, and other factors. Each volume in the series is an authoritative report on the degree of carcinogenic risk
associated with a specific chemical, group of chemicals, industrial process, occupational exposure, lifestyle factor, or
biologic agent. To date, assessments of approximately 900 agents and exposures have been published.
Each assessment is carried out by a working group of international experts who review all published epidemiologic
and experimental data related to the particular agent or exposure. The working group is also charged with indicating
where additional research efforts are needed. Evaluated agents or exposures are assigned to one of five groups
according to the strength of the published scientific evidence for carcinogenicity: Group 1, carcinogenic to humans;
Group 2A, probably carcinogenic to humans; Group 2B, possibly carcinogenic to humans; Group 3, not classifiable as
to carcinogenicity to humans; and Group 4, probably not carcinogenic to humans.
 IARC makes every effort to ensure that the factual material presented in the monographs is reported without bias and
all information is checked meticulously for accuracy. The IARC monographs are recognized as authoritative sources
of information by governments and regulatory bodies worldwide. IARC makes every effort to ensure that the factual material presented in the monographs is reported without bias and
all information is checked meticulously for accuracy. The IARC monographs are recognized as authoritative sources
of information by governments and regulatory bodies worldwide.
NCI has supported the IARC monographs program from its beginning. In addition, NCI represents the United States on
IARC's Governing Council. A portion of IARC's regular budget is provided by the U.S. Department of State.
A complete list of the IARC monographs and up-to-date news about recent assessments
and meetings can be found on the IARC Monographs Web site (http://monographs.iarc.fr).
|
Back to Top
Accelerating Progress in Cancer Prevention
One way to eliminate the suffering and death due to cancer is to develop ways to prevent the disease. Toward
this end, NCI and its international collaborators are seeking to identify medical, behavioral, and environmental
approaches to cancer prevention that can be translated effectively to public health settings. Basic biomedical
research relevant to the prevention of cancer is one avenue of investigation. Exploring strategies to modify
behaviors that can increase a person's risk of cancer - such as poor diet, physical inactivity, excessive sun
exposure, and tobacco use - is a second. How to mitigate the influence of environmental risk factors, including
occupational exposures and infectious agents, is yet another.
The following summaries highlight some of NCI's international activities in the area of cancer prevention.
In 1996, the Director-General of the World Health Organization (WHO) was called upon
to initiate development of a Framework Convention on Tobacco Control (FCTC). The
resulting international convention - the first global health treaty negotiated under the
auspices of WHO - was adopted unanimously by the World Health Assembly in 2003
and entered into force in 2005. Thus far, 126 nations have ratified, accepted, approved,
formally confirmed, and acceded to the treaty.
The WHO's Tobacco Free Initiative (TFI) was established in this context, with the objective
of reducing the global burden of disease and death caused by tobacco. Under the TFI,
the WHO created a scientific advisory committee, called the Scientific Advisory Committee
on Tobacco Product Regulation (SACTob), to provide scientifically sound recommendations
regarding the most effective and evidence-based means to achieve a coordinated regulatory
framework for tobacco products. In 2003, SACTob became a formal study group,
called the Study Group for Tobacco Regulation (TobReg). TobReg provides a formal
mechanism for reporting to WHO's Executive Board in order to draw the attention of
member nations to WHO's efforts in tobacco regulation. NCI scientists have worked with
SACTob/TobReg since 2002 on the development of numerous recommendations aimed at
improving public health and scientific research related to the effects of tobacco use.
 In addition, NCI participated in the TFI effort in 2004 to create
an International Network for Tobacco Testing and Research for
Regulation (INTTARR) to address research issues related to the
development of a global capacity for tobacco product testing and
research. INTTARR, which has since been renamed the Tobacco
Laboratory Network (TobLabNet), is a global network of government,
university, and independent laboratories across the world to
advance research on tobacco product testing. Testing and measuring
tobacco products at the national or regional level are essential
to monitoring compliance by tobacco manufacturers of their
obligations under the FCTC to test and disclose the contents and
emissions of their products. Regulators must also have the capacity
to test and measure tobacco products in order to propose tobacco product content and
emissions regulations in the future. Furthermore, a capacity for testing and research is
one of the factors needed to ensure manufacturers package and label their products in a
manner that does not mislead consumers about the health risks of tobacco.
In addition, NCI participated in the TFI effort in 2004 to create
an International Network for Tobacco Testing and Research for
Regulation (INTTARR) to address research issues related to the
development of a global capacity for tobacco product testing and
research. INTTARR, which has since been renamed the Tobacco
Laboratory Network (TobLabNet), is a global network of government,
university, and independent laboratories across the world to
advance research on tobacco product testing. Testing and measuring
tobacco products at the national or regional level are essential
to monitoring compliance by tobacco manufacturers of their
obligations under the FCTC to test and disclose the contents and
emissions of their products. Regulators must also have the capacity
to test and measure tobacco products in order to propose tobacco product content and
emissions regulations in the future. Furthermore, a capacity for testing and research is
one of the factors needed to ensure manufacturers package and label their products in a
manner that does not mislead consumers about the health risks of tobacco.
Finally, NCI contributes to a multi-agency collaboration, which includes five other institutes
of the National Institutes of Health (NIH), the NIH's Fogarty International Center,
and the TFI, that funds research on tobacco use and related illness in developing countries.
The tobacco industry has exerted substantial influence on tobacco policies throughout the
world, and it is important to understand this influence as we strive to reduce tobaccorelated
disease.
NCI-supported investigators at the University of London's School of Hygiene and
Tropical Medicine are analyzing efforts made by the tobacco industry to influence tobacco
control policies in selected countries, regions, and around the world. These investigators
are using industry documents housed at the Guildford Depository in the United
Kingdom to compile country profiles of tobacco industry activities in 14 countries.
The Guilford Depository was established as a consequence of litigation brought against
several tobacco companies by the State of Minnesota and Minnesota Blue Cross Blue
Shield. The parties settled in 1998, with the agreement of the Minnesota Consent
Judgment, in which the British American Tobacco Company agreed to provide public
access to the internal documents it produced during the discovery process. The documents
were to be made available at the Guildford Depository for a period of 10 years, which
expires in February 2009.
Analysis of the country profiles is offering valuable insights into how the tobacco industry
may have influenced tobacco-related, public policy-making, and scientific research efforts
in the countries being studied. This information is also enabling investigators to examine
the connections between globalization, the tobacco industry, and policy influence, and to
develop recommendations about how to create more effective tobacco-control strategies
and policies to prevent and reduce tobacco use.
In 1999, NCI, the National Institute on Drug Abuse, and the Robert Wood Johnson
Foundation, created the TTURCs to facilitate a transdisciplinary approach to the full
spectrum of basic and applied research on tobacco use. The goal of research projects conducted
through the TTURCs is to help reduce the burden of tobacco-related diseases. In
2004, the National Institute of Alcohol Abuse and Alcoholism joined as a funding partner,
and two TTURCs are currently investigating tobacco use in international populations.
Scientists affiliated with the University of Southern California's Pacific Rim TTURC
have previously studied multiethnic and multicultural populations within the United
States and in China to explore cultural, social, psychological, and environmental factors
that influence tobacco use by adolescents. This research indicated that cultural context
and individual disposition can moderate the effectiveness of prevention programs.
Investigators at the center are now conducting three projects that build on the earlier
findings: 1) a study of substance use by 600 adolescent twin pairs in Southern California
and another 600 adolescent twin pairs in Qingdao, China; researchers will examine
social-environmental and heritable risk and protective factors for tobacco and alcohol
use among the adolescents; 2) a study of why school- and community-based smoking prevention
programs work in some situations and not in others; researchers will study the
effects that a student's cultural and environmental context and dispositional characteristics
(particularly, hostility and depression) have on substance use, and how these factors
influence the effectiveness of prevention and cessation programs; and 3) a study to investigate
the hypothesis that genetic factors responsible for a person's dispositional attributes
(e.g., hostility and depression) may substantially influence both the individual's tobacco
use and the effectiveness of tobacco-control intervention and prevention programs.
The Roswell Park Cancer Institute's TTURC will expand the ongoing International
Tobacco Control Policy Evaluation Survey (ITCPES), which is a longitudinal study of
smokers in the United States, Canada, the United Kingdom, and Australia. The expanded
study will include smokers in Ireland, Thailand, and Malaysia. This expansion will allow
researchers to assess whether tobacco control and prevention policies that are effective
in developed countries are equally effective in developing nations. The researchers will
also conduct follow-up surveys of the 8,300 smokers from the four countries that initially
participated in the ITCPES to evaluate whether comprehensive tobacco control policies
being implemented in these countries are effective in reducing tobacco use. The results of
this TTURC project can provide insights into how and why specific policies influence
tobacco-related behaviors.
|
The prevention of cervical cancer is emerging as a major public health advance; one that has implications for
women throughout the world. Worldwide, cervical cancer causes more than 200,000 deaths each year, and approximately
80 percent of the women who die from this disease live in developing countries. Over the past 30 years,
cervical cancer deaths have declined markedly in the United States - a decline that is due, in large measure, to
effective screening programs. These programs, unfortunately, are quite rare in developing countries. Yet, we now
know that infection with human papillomavirus, or HPV, is the principal cause of cervical cancer in this country
and abroad. This knowledge has made vaccine therapy a viable option for cervical cancer prevention.
Two pharmaceutical companies - Merck and Co, Inc. and GlaxoSmithKline Biologicals (GSK) - have recently
produced vaccines against HPV. Both vaccines are based on technology developed by NCI scientists, whose work
laid the foundation for the production of HPV "virus-like particle," or VLP, vaccines. VLPs contain the L1 outer
coat protein of HPV, yet are noninfectious. They are produced in insect cells or yeast cells by recombinant
DNA technology. The cells make large amounts of the L1 protein, which then self assembles into particles that
look like HPV but do not contain the virus' genetic material.
In a randomized clinical trial, the GSK vaccine - called CervarixTM - provided nearly complete protection against
infections caused by HPV-16 and HPV-18, two strains of HPV that are responsible for 70 percent of all cervical
cancers. The vaccine, which was also highly effective against persistent infections caused by the two strains,
contains a mixture of HPV-16 and HPV-18 VLPs.
Approximately 1,100 women from Canada, Brazil, and the United States participated in the HPV vaccine trial.
These women were randomly assigned to receive doses of the HPV-16/18 vaccine or a placebo initially and then
at 1 month and 6 months after their initial injection. The researchers found that the GSK vaccine was 91.6 percent
effective in protecting against incident infections with HPV-16 or HPV-18 in women treated according to the trial
protocol. The vaccine was 100 percent effective in preventing persistent viral infections during the study period,
and it was 93.5 percent effective in preventing cervical cell abnormalities associated with HVP-16 or HPV-18
infection. Additional follow-up of participants in this trial has demonstrated that effective protection against
infection remains high for up to 4 years. Longer-term efficacy of the vaccine is still not known.
In another clinical trial, the vaccine developed by Merck was found to be nearly completely effective in preventing
incident and persistent infections with HPV-16 and HPV-18 and against cervical cell abnormalities associated with
HPV-16 or HPV-18. This vaccine - called GardasilTM - also protected against two other HPV strains, HPV-6 and
HPV-11, which cause 90 percent of genital warts. More than 12,000 women from 13 countries participated in the
trial. GardasilTM was administered in three doses over 6 months and provided 100 percent protection against
HPV infection for the 18-month duration of the study. The long-term efficacy of the vaccine is still not known, and
follow-up studies will be required to answer this question.
NCI is now conducting a Phase III clinical trial in Costa Rica - where cervical cancer rates are high - to further
test the HPV 16/18 vaccine developed by GSK. Over 7,400 women have been enrolled in the trial. They will be
followed for at least 4 years to allow investigators to gather information about the vaccine's long-term safety and
efficacy. NCI investigators also plan to evaluate other potential effects of the vaccine, including: 1) its effectiveness
against additional HPV strains; 2) its ability to speed the healing of established cervical infections; and 3) evaluate
the immune mechanisms of long-term protection. The NCI trial in Costa Rica will also provide important information
that will be useful to evaluate the cost-effectiveness of HPV vaccination and combined vaccination and screening
prevention efforts.
Harper D, Franco E. Wheeler C, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho N, Roteli-Martins C, Teixeira J, Blatter M, Korn A, Quint W, Dubin G.
Efficacy of a bivalent L1virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled
trial. Lancet, November 13, 2004; 364(9447):1757-1765.
Harper D, Franco E, Wheeler C, Moscicki A, Romanowski B, Roteli-Martins C, Jenkins D, Schuind A, Clemens S, and Dubin G on behalf of the HPV Vaccine Study
group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised
control trial. Lancet, April 15, 2006;367(9518):1213-1290.
Finn S, et al. Prophylactic quadrivalent human papillomavirus (HPV) (types 6, 11, 16, 18) L1 virus-like particle (VLP) vaccine (GardasilTM) reduces cervical intraepithelial
neoplasia (CIN) 2/3 risk. Oral abstract LB-8a. Infectious Diseases Society of America meeting. San Francisco, CA. October 7, 2005.
Villa L, Costa R, Petta C, Andrade R, Ault K, Giuliano A, Wheeler C, Koutsky L, Malm C, Lehtinen M, Skjeldestad F, Olsson S, Steinwall M, Brown D, Kurman R,
Ronnett B, Stoler M, Ferenczy A, Harper D, Tamms G, Yu J, Lupinacci L, Railkar R, Taddeo F, Jansen K, Esser M, Sings H, Saah A, Barr E. Prophylactic quadrivalent
human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre Phase II
efficacy trial. Lancet Oncology, May 1, 2005;6(5):271-278.
|
People can be exposed to two types of phytoestrogens through their diet, isoflavones and
lignans. Isoflavones are found in soy and soy products, and lignans are found in whole
grains, fruits, and vegetables. Some evidence suggests that these agents may protect
against hormone-dependent cancers; however, human studies to date have yielded only
inconclusive results. Large-scale, prospective studies are needed to fully evaluate whether
dietary phytoestrogens are effective in preventing cancer. Such studies, however, are
expensive and difficult to undertake.
NCI is supporting a pilot study, using a subgroup of participants from the European
Prospective Investigation into Cancer and Nutrition (EPIC) project, to determine whether
a large-scale prospective study of phytoestrogens is justified. The EPIC project - the
largest study of diet and health ever undertaken - has registered more than 500,000 men
and women aged 35 to 69 years from 10 different European countries. The pilot study
will examine variations in plasma phytoestrogen levels in 1,600 randomly selected EPIC
participants from 17 different regions across Europe. In particular, plasma levels of the
isoflavones genistein, daidzein, glycetin, and equol and the lignans enterolactone and
enterodiol will be measured. The influence of age, gender, geographic region, and habitual
diet on the plasma levels of these phytoestrogens will also be determined.
Factors associated with reproduction likely play an important role in the development of
ovarian cancer. During pregnancy, the placenta produces large amounts of the female
sex hormones estrogen and progesterone. A number of factors, including gestational age
of the developing fetus, affect the levels of these hormones during pregnancy. Although
research has shown that increasing parity (number of births) reduces a woman's risk of
ovarian cancer, no study to date has examined the associations between indictors of hormonal
exposures during pregnancy and subsequent ovarian cancer risk.
NCI is collaborating with the Karolinska Institute to conduct a large cohort study aimed
at exploring these associations. Investigators are using information gathered on more than
1.2 million women who delivered their first infant between 1973 and 2000 and who
were included in the nationwide Swedish Medical Birth Register. Researchers will: 1)
study ovarian cancer risk using markers of hormone exposures during pregnancy, including
birth weight, gestational age, single or multiple birth, pregnancy-induced hypertensive
diseases, and placental weight; 2) examine whether the protective effects of increasing
parity and a high age at first birth are influenced by markers of hormone exposures
during pregnancy; and 3) assess the importance of other factors on ovarian cancer risk.
The investigators plan to gather information about maternal characteristics, pregnancy
complications, placental weight, and birth characteristics for all births associated
with the women in the cohort. In addition, information about gynecologic surgeries,
vital status, and ovarian cancer incidence for these women will be collected from
population-based registries.
Ovarian cancer accounts for approximately 4 percent of all cancers among women
worldwide and has the highest mortality of all cancers of the female reproductive system.
Use of the Swedish research registries in this study allows a large, cost-effective study
to be conducted where information has been prospectively collected about markers of
hormone exposure during pregnancy.

|
| Unhealthy, High-fat,
High-carbohydrate |
Living a Western lifestyle, which is characterized by a low level of
physical activity and an energy-dense diet rich in easily digestible
(refined) carbohydrates and fats, is associated with an increased risk
of colon cancer. Etiologic models to explain this association have
focused mostly on the effects of diet in exposing the colonic mucosa
to mutagenic or tumor-promoting compounds. The results of this
study should allow formulation of more precise nutritional guidelines
for the effective prevention of colon cancer.
Several NCI-sponsored clinical trials are currently testing the effectiveness of celecoxib
(Celebrex®) in preventing a variety of cancers. Celecoxib, used to treat osteoarthritis and
adult rheumatoid arthritis, reduces inflammation by blocking the activity of the cyclooxygenase-
2, or COX-2, enzyme. The COX-2 enzyme is activated only during inflammation,
and evidence suggests that elevated levels of COX-2 may contribute to the development
of a variety of cancers, including esophageal, stomach, colon, pancreas, liver, breast, lung,
bladder, cervical, and head and neck cancers.
NCI and Pfizer, Inc., jointly sponsored the Adenoma Prevention with Celecoxib (APC) Trial
to investigate whether celecoxib could reduce the occurrence of new colorectal adenomas
(precancerous polyps) in people who already had such a polyp removed. More than 90 centers
- located in the United States, the United Kingdom, Australia, and Canada - and more
than 2,000 men and women age 30 years or older participated in this trial. From November
1999 to March 2002, study participants were randomly assigned to take either 200 mg of
celecoxib twice a day, 400 mg of celecoxib twice a day, or a placebo twice a day for 3 years.
Initial results of the trial were reported in April 2006 and showed that those taking celecoxib
had 33 to 45 percent fewer new adenomas and 57 to 66 percent fewer high-risk adenomas
than those taking the placebo. When adenomas recurred in the participants taking celecoxib,
the growths were fewer and smaller than those in the participants who took the placebo.
In December 2004, use of celecoxib in the APC Trial was suspended because an analysis
by an independent data safety and monitoring board showed that participants taking
the drug had a 2.5-times greater risk of fatal and major non-fatal cardiovascular events
(cardiovascular death, heart attack, stroke, or heart failure) than those on placebo. In
February 2005, the APC investigators published a full analysis of the cardiovascular
events, reporting that celecoxib use for an average of almost three years was associated
with a dose-related increased risk of serious events.
In view of the increased risk of serious cardiovascular events found in the APC trial,
NCI notified all principal investigators of its sponsored trials involving COX-2 inhibitors
and asked them to inform their institutional review boards, data safety and monitoring
boards, and trial participants about this new information. NCI also required that the
informed consent forms for the trials be revised to reflect the new information. Trial
participants were asked to sign new consent forms with updated information about the
risks and benefits of the trials.
A Phase II trial comparing the effectiveness of celecoxib, taken alone or in combination with
eflornithine, in preventing colorectal cancer in patients with familial adenomatous polyposis
(FAP) is currently underway in the United States and the United Kingdom. FAP is an inherited
disorder that is characterized by the development of numerous polyps in the colon and rectum.
People diagnosed with FAP are at increased risk of colon cancer. Although most FAP patients
undergo colectomy (surgical removal of all or part of the colon), researchers are interested in
developing drugs that may offer an additional measure of protection to individuals with this
condition. In the trial, 120 patients between the ages of 18 and 65 who have been diagnosed
with FAP will be randomly assigned to receive celecoxib alone or celecoxib plus eflornithine.
Eflornithine, also known as alpha-difluoromethylornithine, is an inhibitor of the enzyme
ornithine decarboxylase (ODC). Inhibitors of ODC have been shown to suppress tumor
formation in experimental models of bladder, breast, colon, and skin carcinogenesis.
Esophageal squamous cell carcinoma (ESCC) is the third most common cancer of the
digestive tract and the seventh leading cause of cancer-related deaths worldwide. The
incidence of this cancer varies greatly according to geographic location. It is more
common in Northern China, Iran, and the southern regions of the former Soviet
Republic and is less common in Japan, Europe, and Canada. Patients with ESCC are
often diagnosed when the cancer is advanced. However, clinicians have noted that
premalignant lesions can precede the onset of ESCC, and these lesions may represent
a potential target for prevention efforts.
NCI-funded scientists recently completed a randomized, placebo-controlled chemoprevention
trial among people in Lixian, China, who had mild or moderate premalignant disease
and were, therefore, considered to be at high-risk for ESCC. The study participants were
randomly assigned to receive selenomethionine alone, celecoxib alone, a combination of
selenomethionine and celecoxib, or a placebo over a period of 10 months. A total of 360
individuals were randomized to the four groups; 238 individuals were included in the
final analysis. The results of the trial were reported in 2005.
Overall, there was a trend toward increased regression and decreased progression of
premalignant lesions in selenomethionine-treated subjects in comparison with those not
treated with selenomethionine, but the results were not statistically significant. In
unplanned analyses, treatment with selenomethionine favorably affected a change in
dysplasia grade among the 115 subjects who had mild premalignant disease at baseline
but not among the 123 subjects who had moderate premalignant disease at baseline.
Treatment with celecoxib had no effect on disease regression or progression. This is the
first report of a potential chemoprevention agent for ESCC.
|
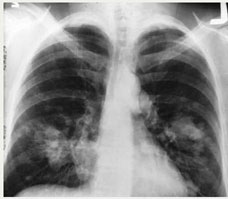 |
| This is an x-ray image
of a chest. Both sides
of the lungs are visible
with a growth on the
left side of the lung,
which could possibly
be lung cancer. |
As the number of former smokers increases, so does the need to find
effective interventions for preventing lung cancer in this high-risk population.
Inhalers have long been considered a safe and effective way to
deliver medications for treating chronic asthma. Now, scientists are testing
whether inhalers can safely and effectively deliver agents to prevent
lung cancer. Scientists are now evaluating whether inhaled budesonide,
a steroid commonly used to treat asthma, can prevent the development
of lung cancer.
In preclinical studies, NCI investigators found evidence that inhaled
budesonide may impede lung tumor growth if it is given in the early
stages of tumor development. The studies showed that glucocorticoids,
which include budesonide, were nearly 90 percent effective in preventing
lung adenomas in mice. These results served as the foundation for a Phase II clinical trial, in
which a group of Canadian smokers took inhaled budesonide for 6 months. In this trial, which
was reported in 2004, the drug had no effect on the growth of bronchial lesions or the prevention
of new lesions. However, spiral computed tomography (CT) scans performed on the trial participants
revealed that budesonide may have affected small nodules - some of which may have
been precancerous - in the peripheral lungs.
On the basis of this observation, a new clinical trial testing inhaled budesonide recently began
in Italy. This Phase II trial, supported by NCI and led by the European Institute of Oncology, will
enroll individuals who are already receiving annual spiral CTs as part of a larger lung cancer
screening trial. The researchers will focus on individuals who, after the second annual CT, have
persistent lung nodules that may be precursors to lung adenocarcinomas. These individuals will
be treated for 1 year with either budesonide or a placebo. If the budesonide is found to be an
effective chemopreventive agent for the trial participants - that is, it causes lung nodules to
regress - a larger trial will follow. The investigators will also assess whether this drug is safe
for those who are at higher risk of developing lung cancer.
Inhalers have the advantage of delivering medication directly to the lungs, limiting the risk of
potential side effects in other parts of the body. Because it is a targeted delivery system, it can
reduce overall toxicity. However, an agent to prevent lung cancer will probably need to be taken
over an extended period of time, therefore the long-term toxicity of the agent is a critical concern.
|
In 2003, NCI, the Centers for Disease Control and Prevention, and the Substance Abuse
and Mental Health Services Administration, jointly developed and launched a Web portal
called Cancer Control PLANET (Plan, Link, Act, Network with Evidence-based Tools).
This Web portal serves as a doorway to new evidence-based tools - developed through a
public-private effort involving these federal agencies and the American Cancer Society -
that can help communities better understand and address their cancer burden. Cancer
Control PLANET is organized around five steps that U.S. communities can take to develop
a comprehensive cancer control plan.
A prototype of an international version of the Cancer Control PLANET Web portal will
be demonstrated at the 2006 International Cancer Control Conference, in conjunction
with the UICC World Cancer Congress in Washington, D.C. Full implementation of the
International Cancer Control PLANET Web portal is anticipated in 2007, with ongoing
NCI involvement in updating international data for the portal in future years.
Back to Top
Improving Early Detection and Diagnosis
For nearly all cancers, treatment options and survival are related to the stage of disease at diagnosis. The prognosis
is generally better and treatment usually more successful if the disease is detected and diagnosed early while still
localized. Unfortunately, many cancers have no symptoms at early stages and are not detected until the disease is
advanced. Methods to detect and diagnose cancer include imaging procedures and laboratory tests. Laboratory tests
may identify cancer cells (e.g., urine cytology for bladder cancer), specific biomarkers (e.g., the KIT receptor protein
for gastrointestinal stromal tumors), or, more recently, distinctive gene-expression microarray patterns (e.g., the
Lymphochip for diagnosing different types of lymphoma; see The Leukemia and Lymphoma Molecular Profiling Project).
NCI actively invests in biomarker development programs and in research toward the development of advanced
technologies for cancer detection and diagnosis. Some of these efforts are conducted in international laboratories
and medical institutions.
One NCI-supported effort in this area is the Early Detection Research Network (EDRN) to identify early cancer
biomarkers (described below). Other NCI-supported efforts that involve international collaborators and seek
to improve methods for the early detection and diagnosis of cancer are described here.

|
| DNA microarray technology
is a powerful
new research tool that
allows scientists to
assess the level of
expression of a large
subset of the 100,000
human genes in a
cell or tissue. This
technology can quickly
produce a snapshot
of the genes that are
active in a tumor cell,
critical information in
narrowing the precise
molecular causes of
a cancer. |
NCI established the Program for the Assessment of Clinical Cancer
Tests (PACCT) to ensure that promising cancer biomarkers are
appropriately evaluated for clinical usefulness. A critical barrier to
advancing cancer diagnostics is the lack of reference tissues for
evaluating promising biomarkers. NCI identified two valuable collections
of tissue specimens at the Institut Municipal d'Investigació
Mèdica (IMIM) in Barcelona, Spain and the British Columbia
Cancer Agency (BCCA) in Canada. The IMIM specimens are from
bladder cancer patients diagnosed in five areas of Spain, and the
BCCA specimens are from Canadian ovarian cancer patients.
Although the BCCA microarrays are still under construction, the IMIM arrays are
currently available. The IMIM microarrays are statistically designed to address major
research questions in bladder cancer.
The University of Tokyo is home to one of the world's premier fluorescence imaging
laboratories, and NCI scientists are working with Japanese researchers to develop new
activatable imaging probes for discovering cancers at an early stage. Activatable optical
probes produce a signal and become detectable only after they reach their target. This new
method can detect very small cancer nodules with very high sensitivity compared to current
imaging methods, and it may also provide improved specificity over current methods.
Magnetic resonance imaging is emerging as the most effective diagnostic imaging tool
for visualizing the anatomy and pathology of the prostate. NCI scientists are working
with researchers at the Princess Margaret Hospital in Toronto to improve a magnetic
resonance imaging-guided prostate biopsy system and to design the next generation of
these devices. In addition, NCI is examining targeted biopsy specimens of prostate tissue
to identify the next generation of molecular targets for diagnosis and therapy.
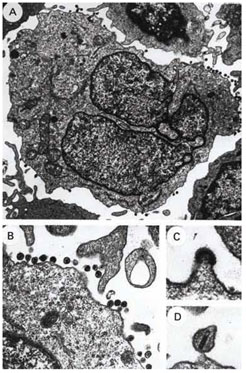
|
| Images of Blood
Leukocytes from an
AIDS Patient
Producing HIV |
To encourage research on acquired immunodeficiency syndrome
(AIDS) and cancer, NCI established the AIDS and Cancer Specimen
Resource (ACSR). This international resource for tissue and biological
samples serves researchers working in the fields of AIDS, cancer,
virology, immunology, pathology, epidemiology, tumor biology, and
assay development, as well as others. The ACSR is a repository of
human immunodeficiency virus-1 (HIV-1)-infected materials from
a wide spectrum of HIV-related or associated diseases and from
appropriate HIV-negative controls. Special sets of specimens include
serial samples from patients undergoing treatment in clinical trials.
More than 100,000 individual specimens are contained in the repository,
including samples from Africa, Brazil, Russia, and Thailand.
NCI investigators are working with researchers at the University of
Nebraska Medical Center in Omaha to define the gene expression
profiles of all types of human lymphoid malignancies. The project is supported by an international
collaboration involving investigators from the Southwest Oncology Group; the
British Columbia Cancer Agency in Vancouver, Canada; the Norwegian Radium Hospital
in Oslo, Norway; the University of Würzburg in Würzburg, Germany; the University of
Barcelona in Barcelona, Spain; and St. Bartholomew's Hospital in London, England.
The LLMPP uses "Lymphochip" cDNA (complementary DNA) microarrays, which are
enriched in genes that are expressed in and/or function in lymphocytes. Lymphochip
microarrays allow measurement of the RNA expression levels of the represented genes.
Gene expression profiles developed as a result of this project may someday be used for
disease classification (diagnosis), prognosis, and therapy selection. Already, results from
the LLMPP indicate that these profiles can improve diagnostic accuracy and provide
prognostic information.
The LLMPP is initiating a multicenter clinical trial to evaluate a lymphoma diagnostic
chip, called LymphDX, which was designed by the company Affymetrix in collaboration
with NCI researchers using LLMPP data. The study will demonstrate the feasibility of
disseminating the LymphDX microarray technology to all of the participating sites and
will also evaluate the diagnostic utility of the LymphDX chip in a prospective study.
The American-Russian Cancer Alliance (ARCA), established in 2001, is a consortium of
American and Russian cancer research institutes that conducts scientific research and medical
education conferences both in the United States and Russia. The participating institutes
include the University of Maryland Greenebaum Cancer Center in Baltimore, Maryland;
the Fox Chase Cancer Center in Philadelphia, Pennsylvania; and the Kurchatov Institute
and the N.N. Blokhin Cancer Research Center in Moscow, Russia. The Fox Chase Cancer
Center is an NCI-designated Comprehensive Cancer Center, while the Kurchatov Institute
is Russia's premier nuclear research center. Among ARCA's programs is a groundbreaking
effort funded by the United States to use Russia's expertise and nuclear facilities to produce
radioisotopes for diagnostic and therapeutic applications in oncology.
In one project, scientists at the Fox Chase Cancer Center have been developing agents
for positron emission tomography (PET) detection and staging of cancer using iodine-124
from the Kurchatov Institute. In another study, University of Maryland researchers are
investigating novel ways to use isotopes to destroy blood vessels that feed malignant
tumors. Their initial research focused on actinium-225, but now they are working with
another isotope, polonium-210. In a third project, Fox Chase Cancer Center investigators
are studying the use of bismuth-213 for the radiotherapy of solid tumors.
NCI provides support for the infrastructure of ARCA through a supplement to the
Cancer Center Core Grant awarded to the Fox Chase Cancer Center. The Institute is
also providing funding for a tobacco research grant involving Fox Chase and the N.N.
Blokhin Cancer Research Center through the Fogarty International Center's International
Tobacco and Health Research and Capacity Building Program. In March 2006, NCI
supported a major conference in Moscow that was organized by three ARCA partners
(the Fox Chase Cancer Center, the University of Maryland Greenebaum Cancer Center,
and the N.N. Blokhin Cancer Research Center) and entitled "Prevention and Treatment
of Tobacco-Related Cancers." Approximately 200 Russians attended this conference,
including a representative of the Russian legislature, as well as the U.S. Ambassador to
the Russian Federation. Several NCI scientists were invited speakers. In Autumn 2006,
NCI will host a workshop, involving ARCA partners, in Bethesda, Maryland, on the use
of isotopes in cancer diagnosis and treatment.
In conjunction with ARCA, NCI's Office of International Affairs (OIA) is sponsoring
a 1-year (2006-2007) training visit of a Russian scientist from Lomonosov Moscow
State University to the University of Maryland School of Medicine's Division of Nuclear
Medicine. In 2005, OIA sponsored shorter training visits of three Russian scientists to the
same institution. In February 2005, NCI sponsored a visit of three representatives from
the N.N. Blokhin Cancer Research Center to various cancer research centers and governmental
and non-governmental organizations in the United States, including NCI. The purpose
of the visit was to observe "best practices" in cancer communications to be adapted
in Russia. Also in 2005, NCI sponsored six Russians to participate in the Institute's
Summer Curriculum in Cancer Prevention and Control
which is described
in NCI's Summer Course on the Principles and Practice of Cancer Prevention and Control.
In 2004, the third EORTC-NCI International meeting to discuss cancer molecular markers
drew more than 200 participants from Europe, Asia, Africa, and the United States.
NCI staff members were involved in planning the meeting and participated as session
chairs and speakers. During the meeting, staff from the NCI and the U.S. Food and Drug
Administration and members of the EORTC developed and presented a 1-day tutorial for
industry on pathways for development of clinical laboratory tests using cancer molecular
markers. The next international EORTC-NCI meeting on cancer markers is scheduled to
be held in September 2006.
|
In 2000, NCI formed the Early Detection Research Network (EDRN), a consortium of government,
academic, and private-sector institutions focused on developing, evaluating, and validating biomarkers
for early cancer detection and risk assessment. Today, the more than 300 researchers
and 40 institutions that make up the EDRN are at the forefront of technology-driven research on
the use of biomarkers for the early detection of cancer.
NCI is funding many laboratories that develop biomarkers, including several overseas. One
example is the laboratory of Dr. Zvi Livneh at the Weizmann Institute of Science in Rehovot,
Israel. This laboratory is investigating whether detecting lower-than-normal activity of DNA
repair enzymes in blood cells can be used as a biomarker for lung cancer risk in smokers.
Another example is the laboratory of Dr. Bruce Robinson of the University of Western Australia in
Perth, Australia. This laboratory has identified a novel protein that shows promise as a biomarker
for mesothelioma. Work performed in Dr. Robinson's laboratory is part of a multicenter study that
will evaluate and validate several biomarkers for this deadly disease.
Once biomarkers are identified, they must be validated and undergo testing in large clinical trials
with human participants. Ultimately, the lab tests that result from EDRN research will be added to
the clinician's toolbox to aid in cancer prevention and in early therapeutic intervention.
The Web site for the EDRN is located at http://edrn.nci.nih.gov/.

|
| Dr. Zvi Livneh |

|
| Dr. Bruce Robinson |
|
Back to Top
Developing Effective and Efficient Treatments
Approximately one third of all cancers are avoidable through lifestyle changes. These changes include: stopping
smoking, maintaining a healthy weight, being physically active, eating a moderate-fat diet with enough fruits and
vegetables, avoiding too much alcohol, and protecting the skin from harmful sun exposure. Enough information is
also available to permit the early detection and effective treatment of another one third of cancer cases. Some of
the most commonly occurring cancer types are curable with existing treatments. Nonetheless, millions of cancer
cases worldwide cannot currently be prevented or cured. Therefore, we must continue to develop new and
effective treatments.
Developing new therapies for cancer is central to much of the research supported by NCI. By partnering with
research institutions and investigators around the world, we should be able to accelerate the pace of development
of new drugs and other treatments and shorten the time required to recruit patients into and complete clinical trials.
In Europe, NCI created a liaison office to coordinate many of its research and treatment programs conducted
with European collaborators (see article on the
NCI Liaison Office). More examples of global
NCI-supported programs and efforts to develop new, effective, and efficient treatments for cancer are described
in this section.
NCI is assisting international research institutions with clinical trial investigations of targeted
radiation therapy - also known as radioimmunotherapy - by supplying overseas scientists
with the necessary reagents and expertise pertaining to their use. One clinical trial
that is currently being conducted at the German Cancer Research Center in Heidelberg,
Germany is evaluating a monoclonal antibody conjugated to the radioisotope bismuth-213
for the treatment of NHL. The monoclonal antibody targets a protein antigen called
CD20, which is found on NHL cells. The German researchers obtained the reagents they
needed for this trial from NCI through a Material Transfer Agreement with the Institute.
The NCI supports research projects at several Canadian universities, cancer centers, and
hospitals, as well as clinical trials run by Canadian cooperative groups. One group that
receives NCI support is the Princess Margaret Hospital Phase II Consortium. This consortium
is the only non-U.S.-based trial group funded by NCI to carry out Phase II studies of
new anticancer drugs. In addition, the National Cancer Institute of Canada is a participant
in the NCI Clinical Trials Cooperative Group Program. Individual Canadian institutions
are also members of other NCI-supported cooperative groups, including the
American College of Surgeons Oncology Group, the Cancer and Leukemia Group B, the
Children's Oncology Group, the Eastern Cooperative Oncology Group, the Gynecologic
Oncology Group, the National Surgical Adjuvant Breast and Bowel Project, the North
Central Cancer Treatment Group, the Radiation Therapy Oncology Group, and the
Southwest Oncology Group. More than 90 percent of the investigational drugs shipped
internationally by NCI's Cancer Therapy Evaluation Program (CTEP) currently go to
Canadian investigators (see related article on CTEP).
Routine, complete lymphadenectomy - the surgical removal of regional lymph nodes -
for patients with clinically localized primary melanoma remains controversial because
most patients will have tumor-free nodes. A minimally-invasive procedure called intraoperative
lymphatic mapping and biopsy of the sentinel lymph node is a promising alternative.
Examination of the sentinel lymph node - defined as the regional node most likely
to contain cancer cells spreading from the primary tumor - can identify patients with
occult (microscopic) metastasis. These patients should undergo complete lymphadenectomy,
while other patients do not require further nodal assessment.
The Multicenter Selective Lymphadenectomy Trial (MSLT-I), headed by investigators from
the John Wayne Cancer Institute in Santa Monica, California, is comparing two treatments
for early melanoma: 1) wide excision plus sentinel node biopsy (SNB), followed
immediately by complete lymphadenectomy if the sentinel node contains cancer; and
2) wide excision, followed by complete lymphadenectomy only for clinical evidence of
nodal metastasis during postoperative observation.
MSLT-I, the largest randomized surgical trial for melanoma, enrolled 338 patients from
North America, 630 from Europe, and 1,033 from Australia. International enrollment
allowed rapid accrual. Interim results, which were reported in 2005, showed that SNB
performed by doctors with adequate training and experience can identify occult nodal
metastases with 95 to 97 percent accuracy. The interim data also indicate that SNB significantly
lengthens the disease-free survival of all patients and prolongs the overall survival
of patients with occult nodal metastases.
Because these findings strongly support early lymphadenectomy for occult nodal metastasis,
MSLT-I data are changing the standard of care. A follow-up study, MSLT-II, is now
open to patient accrual and will enroll at least 1,925 patients in another international
collaboration of melanoma centers.
|
| Scanning Electron
Microscope Picture of
a Breast Cancer Cell |
An international clinical trial led by the National Cancer Institute of
Canada Clinical Trials Group (NCIC CTG), with support from the
Canadian Cancer Society (CCS), and in partnership with NCI and
Novartis Pharmaceuticals, showed that the estrogen-suppressing
drug letrozole (Femara®) reduced the risk of breast cancer recurrence
and the incidence of new breast cancer in the opposite breast by
42 percent compared to placebo in women whose tumors were
hormone receptor-positive.
Previous studies had shown that 5 years of treatment with the drug tamoxifen after
surgery, radiation therapy, and chemotherapy for early-stage breast cancer could reduce
the risk of recurrence by almost half in women whose tumors were estrogen receptorpositive.
Some of these studies also showed, however, that no additional benefit is
obtained by continuing tamoxifen treatment beyond 5 years. Because more than
50 percent of the women who experience a recurrence of their cancer do so more than
5 years after diagnosis, additional treatment options are necessary. This trial showed
that a 5-year course of letrozole, when given after 5 years of tamoxifen therapy,
significantly reduced the risk of local recurrence and metastasis (distant recurrence).
In addition to patients recruited through the NCIC CTG and NCI, European participants
were enrolled in the letrozole study by the European Organization for Research and
Treatment of Cancer (EORTC) and the International Breast Cancer Study Group
(IBCSG). Participants in the trial were enrolled through hospitals, cancer centers, and
institutes throughout Canada, the United States, England, Belgium, Ireland, Italy, Poland,
Portugal, and Switzerland. The trial's participants will continue to be followed for 10 to
15 years.

|
| NCI Frederick Cancer
Research and
Development Facility
Prepares Samples of
Natural Substances
for Chemical Analysis |
Between 1986 and 2004, NCI's Developmental Therapeutics Program
(DTP) acquired plants and continues to collect marine organisms to
screen for potential anticancer compounds through collection agreements
with over 25 tropical and subtropical countries. More than
50,000 plant specimens were collected in Africa and Madagascar,
Central and South America, and Southeast Asia. More than 13,000
specimens of marine invertebrates and marine algae have been collected
to date, initially from the Indo-Pacific region, and, since 2002,
from areas worldwide.
In undertaking these collections, NCI committed itself to the conservation
of biological diversity, as well as to policies of fair and equitable
collaboration and compensation in interacting with the source countries
participating in the collection programs. Agreements based on the
NCI Letter of Collection (LOC) or Memoranda of Understanding
(MOUs) have been signed with relevant government organizations in
many of the source countries. The first such agreement was signed with Madagascar in
1990, 3 years before an international conference in Rio de Janeiro, Brazil, that led to the
signing of the Convention on Biodiversity (CBD). In addition, the NCI model agreements
have formed the basis for many international accords not involving the Institute or the
United States and are still used as a basis for discussion by many organizations wishing
to conduct biodiscovery programs.
More information about DTP's international outreach initiatives is found
in NCI's Developmental Therapeutics Program.
|
Since its inception, NCI's Cancer Therapy Evaluation Program (CTEP) has provided promising anticancer drugs to
international researchers for both preclinical and clinical studies. In 2004 alone, new investigational drugs were
shipped to, among others, Australia, Canada, China, Germany, Hong Kong, Peru, and the United Kingdom for clinical
trials, special exceptions ("compassionate use") treatment, and laboratory research.
Most drugs are provided under CTEP to investigators who are collaborating on NCI-sponsored clinical trials led by
investigators in the United States. International participation in clinical trials allows for increased patient accrual,
more rapid trial completion, and access to patients with cancers that may be rare in the United States. For studies
led by foreign investigators, both the trial concept and protocol are reviewed in detail by NCI before an agreement
is reached.
Requests to CTEP for "compassionate use" access to experimental drugs are reviewed on a patient-by-patient basis,
and all approved patients must meet the same eligibility criteria as patients in the United States.
Currently, NCI is also providing investigational new drugs developed by CTEP to foreign investigator-led trials in both
Europe and Asia. Three trials in the United Kingdom, sponsored by Cancer Research U.K., are testing the investigational
compounds provided by CTEP. In addition, CTEP is providing experimental drugs for several trials conducted by
the Cancer Therapeutics Research Group in the Far East, comprised of investigators in Hong Kong, Singapore, Korea,
and Australia.
Many existing collaborative relationships under CTEP are the result of NCI's strong training programs, which invite
international scientists to study at NCI and take what they have learned back to their home countries (see
NCI's Visiting Scientist Programs).
These investigators often choose to maintain the professional relationships developed during their time at NCI, and
they become active participants in international studies.
In addition, many NCI-sponsored Clinical Trials Cooperative Groups that use investigational new drugs developed by
CTEP have international investigators as members. Such groups include the National Surgical Adjuvant Breast and
Bowel Project, the Eastern Cooperative Oncology Group, and the Children's Oncology Group.
|
|
| Three Strains of Mice
Used in Experiments |
NCI is collaborating with investigators at the
Peter McCollum Cancer Centre in Melbourne,
Australia and Juntendo University in Tokyo,
Japan to explore combination treatment of
a number of mouse tumor types with bortezomib
(Velcade®) and an agonist antibody
against the Apo2L/TRAIL "death receptor."
As part of the collaboration, the Australian
and Japanese investigators have supplied NCI
with a quantity of the purified antibody and
with lymphocyte - tumor hybrid cells called
"hybridomas" that produce it.
The Apo2L/TRAIL receptor is one of several members of the tumor necrosis factor
superfamily of receptors that are able to induce apoptosis (programmed cell death)
when activated. This receptor has received considerable attention lately because of the
finding that many cancer cell types are sensitive to Apo2L/TRAIL-induced apoptosis,
whereas most normal cells are not.
Bortezomib works by blocking the action of structures inside cells called "proteasomes,"
which are large enzyme complexes that degrade abnormal or misfolded proteins and
proteins that are normally targeted for destruction. Some proteins are normally targeted
for orderly destruction to maintain cellular growth control. Research has shown that
proteasome inhibition can induce apoptosis and that cancer cells are more sensitive to
this effect of bortezomib than normal cells.
The antibody against the Apo2L/TRAIL receptor alone has shown some efficacy against
tumor cells, but preliminary experiments have indicated that bortezomib enhances its
activity. This combination treatment will hopefully allow a reduction of tumor burden in
the absence of the major immunosuppression that can occur during traditional chemotherapy
or radiation therapy. The investigators' aims are to promote local tumor destruction
and, hopefully, to improve the natural immune response against the tumor cells.
|
Since 1955, NCI's Developmental Therapeutics Program (DTP), part of the Division of Cancer Treatment and Diagnosis,
has been tasked with planning, conducting, and facilitating the discovery and development of new therapeutic agents
for cancer.
One service of DTP that is open to investigators throughout the world is a screening program that can test new
compounds for anticancer activity both in the "test tube" (in vitro) and in animal models (in vivo). Among the more
than 24,000 compounds accepted for screening by DTP since the beginning of 2000, approximately 64 percent came
from international institutions.
For many years, DTP has also formally collaborated in drug development with the European Organization for Research
and Treatment of Cancer, Cancer Research U.K., and the Southern Europe New Drug Organization in Italy.
For example, DTP's Pharmaceutical Resources Branch and Toxicology and Pharmacology Branch have participated in
the formulation and evaluation of the pharmacokinetics and toxicity of benzothiazole and its analogs to determine the
suitability of these compounds for testing in early clinical trials. DTP also carried out the formulation, development,
and production of clomesone, bryostatin, rhizoxin, and SJG-136, which had their initial clinical evaluations in Europe.
A number of anticancer agents in development by DTP originally came from Japan. One of these, a benzoylphenylurea
produced by a Japanese chemical company, involved very active joint research in which the company provided
a number of prodrugs to NCI. Prodrugs are inactive compounds that the body can metabolize to become active drugs.
In addition to collaborations with larger organizations, DTP works directly with investigators in Europe and Japan
who have expertise in the selection of candidate agents for screening. Current projects include the development
of benzothiazoles and pyrrolobenzodiazepines (United Kingdom) and aminoflavone derivatives (Japan). These
compounds are currently in early clinical trials in those countries.
|
Following a successful pilot study, NCI is supporting a cooperative agreement to develop
an ICTCM that partners the University of Texas M. D. Anderson Cancer Center with
the Cancer Hospital, Fudan University in Shanghai, China. Researchers affiliated with
the center will investigate the benefits of some traditional Chinese medicine (TCM)
treatments for cancer patients.
During the 2-year pilot study, which began in 2003, the ICTCM investigated three
categories of TCM: 1) herbal and natural treatments that target the disease and related
symptoms; 2) acupuncture for some side effects of cancer treatment; and 3) the biobehavioral
effects of qigong, a self-healing art that combines movement and meditation, and
other mind/body interventions. This work is being advanced further under a new 4-year
cooperative agreement.
While the NCI has supported research in individual topics related to TCM, such as
acupuncture, these are the first NCI grants to support the development of an international
partnership to study multiple aspects of TCM.

|
| Dr. Yosef Yarden |
NCI has named Dr. Yosef Yarden of the Weizmann Institute of Science in Rehovot,
Israel as a MERIT Award recipient for his project titled "Next Generation Strategies to
Intercept ErbB Signaling." The MERIT Awards were established to provide experienced
investigators with long-term, stable support to foster their continued creativity.
Dr. Yarden is investigating the epidermal growth factor receptor (EGFR/ErbB) family of
proteins with the goal of designing therapies that inhibit their oncogenic properties. He has
been involved in many crucial developments in this field, including isolating EGFR/ErbB-1,
identifying several proteins that bind to EGFR/ErbB-1, and understanding the role of another
member of the EGFR family (HER2/ErbB-2) in cell signaling and tumor development.
In his MERIT-funded research, Dr. Yarden will focus on developing therapies that target
ErbB. This work will be carried out through development of: 1) novel molecules that bind
EGFR/ErbB receptors; 2) engineered soluble portions of these receptors; and 3) inhibitors
of EGFR/ErbB receptor activation that promote receptor degradation. Immunotherapy
strategies will also be explored.
|
The NCI Liaison Office, located in Brussels, Belgium, coordinates several of the Institute's research and treatment
programs in Europe through formal agreements with organizations such as the European Organization for Research
and Treatment of Cancer (EORTC), Cancer Research U.K. (CRUK), and the Southern Europe New Drugs Organization
(SENDO). Additional Liaison Office activities involve less formal interactions with other European cancer research
organizations, institutes, laboratories, and pharmaceutical/chemical corporations.
The primary goal of the Liaison Office is to foster exchanges of information, experimental drugs, research protocols,
scientists, and scientific expertise between NCI and its European collaborators. The Liaison Office participates in
numerous European committees and working groups involved in new drug development. It also provides representation
to the EORTC Board and Council and the CRUK Phase I/II clinical trials committee. In addition, the Office has
observer status with the European Drug Development Network (EDDN) and supports an international exchange of
experimental drugs for preclinical and clinical evaluation.
The NCI Liaison Office also collects European cancer clinical research protocols and coordinates their inclusion in
NCI's Physician Data Query (PDQ®) cancer information database, the contents of which are made available worldwide
through various channels (see description of PDQ®).
A collaborative relationship has also been established between the Liaison Office and the International Network
for Cancer Treatment and Research (INCTR), which is also based in Brussels (for more information on
INCTR,
see International Network for Cancer Treatment and Research).
Finally, the Liaison Office serves as the European hub for the TELESYNERGY® Medical Consultation WorkStation,
which was developed by the National Institutes of Health's Center for Information Technology and is deployed by
NCI. TELESYNERGY® is an integrated telecommunications system of computers, microscopes, cameras, and other
equipment that can transmit X-rays and other medical images or a live examination of a patient to distant sites,
where clinicians can discuss the case as if they were in the same room (see more about this program).
|
The AIDS Malignancy Consortium (AMC) is an NCI-supported clinical trials group
founded in 1995 to support innovative trials for AIDS-associated malignancies, including
non-Hodgkin lymphoma, primary central nervous system lymphoma, Kaposi sarcoma,
and cervical cancer. The International Working Group of the AMC - one of five AMC
working groups - is focused on developing partnerships with international investigators
to pursue non-myelosuppressive, hypothesis-driven clinical trials for AIDS-associated
malignancies that are suitable for evaluation in resource-poor settings.
In one such clinical trial, researchers with the East Africa-Case Western Reserve
University Research Collaboration confirmed that dose-modification of oral chemotherapy
in the management of AIDS-related non-Hodgkin lymphoma results in less toxicity
and does not compromise efficacy. Future AMC clinical trials will include international
sites in areas most affected by the global AIDS pandemic, such as Uganda, Zambia,
Kenya, Tanzania, and Brazil.
Back to Top
Understanding the Factors that Influence Cancer Outcomes
NCI is dedicated to improving cancer-related outcomes and reducing the impact and burden of cancer on patients,
their families, and society. The Institute seeks to identify, measure, and understand factors that influence the
quality and effectiveness of cancer-related programs and care both in the United States and abroad. These factors
may be biological, behavioral, sociocultural, environmental, or economic in nature. Cancer-related outcomes
can be measured in terms of: intervention efficacy; survival; health-related quality of life; satisfaction with care;
performance of the health care system; and the economic burden on individuals, families, or society at large.
For clinicians and patients, cancer outcomes research can provide evidence about the benefits, risks, and results of
interventions, which can then be used to make more informed decisions. For health care managers and purchasers,
cancer outcomes research can identify potentially effective strategies that can be implemented to improve the
quality and value of cancer-related care.
The following are examples of NCI research with international partners into possible factors that can influence
cancer-related outcomes. Of special note is the NCI's involvement with the International Breast Cancer Screening
Network (IBSN), which is described below.
It is difficult to directly assess the impact of cancer-related interventions on a population
level. Mathematical modeling, however, may be an appropriate method for performing
such assessments. Researchers at the Erasmus University Medical Center, Department of
Public Health (Rotterdam, The Netherlands) have developed a microsimulation model,
called MISCAN-COLON, to evaluate the population benefit and expenditures for interventions
to reduce the burden of colorectal cancer.
Using MISCAN-COLON, a large number of individual life histories can be simulated in
each of which several colorectal lesions can emerge. Screening for colorectal cancer can
then be simulated, which will alter some of the life histories. Multiple factors can be
specified in the MISCAN-COLON model, including: demographic, epidemiologic, and
economic information; the characteristics of screening; and other information, such as
risk factors, treatment practice, and behavioral aspects. Various assumptions about the
natural history of colorectal cancer and screening and about surveillance strategies can
also be incorporated in the model. MISCAN-COLON produces a detailed output of
colorectal cancer incidence, prevalence, and mortality, as well as the results and effects
of screening. It can be used to test hypotheses against empirical data. The model can also
be used for evaluating screening policies and for choosing between competing policies
by comparing their simulated costs and effectiveness outcomes.
The Erasmus University Department of Public Health and NCI are working with
researchers in Europe, Canada, and the United States to conduct additional validation
studies of MISCAN-COLON and to use the model for program and policy evaluation.
In 2004, MISCAN-COLON was used to evaluate the colorectal cancer screening
coverage policy of the U.S. Center for Medicare and Medicaid Services.
Erasmus University is also a partner in an NCI-funded Cancer Intervention and
Surveillance Modeling Network (CISNET) grant on colorectal cancer, and MISCANCOLON
was used in the CISNET evaluation of the U.S. Department of Health and
Human Services' Healthy People 2010 goals.
The CIBMTR is a research program funded by a grant from NCI that collects detailed
data on blood and bone marrow transplant patients from more than 450 transplant
centers in 48 countries. Formed in 2004, the center helps researchers share patient data
and conduct scientific studies on transplant treatments for cancer. The CIBMTR collects
data on about 65 percent of the allogeneic hematopoietic cell transplants (HCTs) done in
North and South America, about 35 percent of the allogeneic transplants done elsewhere,
and about 60 percent of autologous HCTs done in North and South America. The center's
database contains information on more than 100,000 blood and marrow transplants.
The ability to perform retrospective analyses on worldwide transplant data has led to a
number of important conclusions regarding transplant patient outcomes, including the
following:
Chronic graft-versus-host disease (and its treatment) increases the risk of developing
squamous cell carcinoma of the skin and of mucosal surfaces, and bone marrow
transplant patients should be screened for this complication.
Human leukocyte antigen (HLA)-mismatched umbilical cord blood should be considered
as a source of transplantable hematopoietic cells for patients who do not have an
HLA-matched adult donor.
Ethnicity affects the risk of developing acute graft-versus-host disease, but not overall
survival after transplantation.
Although peripheral blood stem cell transplants appear to be at least as successful as
marrow transplants in adults, this is not the case in children. Children who received
peripheral blood transplants showed higher rates of chronic graft-versus-host disease
and treatment-related mortality, as well as lower survival.
Treating rectal adenocarcinoma tumors preoperatively with chemoradiotherapy (i.e.,
chemotherapy combined with radiation therapy), results in a wide spectrum of responses
ranging from complete responsiveness to complete resistance. NCI has collaborated with
researchers at University Hospital and Medical School of the University of Göttingen,
Germany in a study to investigate whether parallel gene expression profiling of the
primary tumor can help stratify patients into groups of likely responders and likely
non-responders. The results of the study suggest that pretherapeutic gene expression
profiling may be helpful in predicting the response of rectal adenocarcinomas to
preoperative chemoradiotherapy.
NCI and the Drug Information Association (DIA) cosponsored a conference in 2004
that focused on innovative techniques for patient-reported outcomes assessment based on
item-response-theory modeling and computerized adaptive testing. The conference goal
was to improve the quality and feasibility of measuring patient-reported outcomes, with
an emphasis on cancer. DIA is a nonprofit organization with 27,000 individual members
from 75 countries. Its membership is drawn from the pharmaceutical industry, academia,
government, and contract research organizations.
At the meeting, NCI and DIA member scientists outlined a research agenda for the future
of health outcomes and behavioral science measurement, including use of computerized
adaptive testing for measuring key health domains, such as physical functioning, emotional
well-being, fatigue, and pain.
|
NCI is a leading member of IBSN, a voluntary consortium involving 25 countries that have active,
population-based screening mammography programs. IBSN encourages collaborative research
to identify and foster efficient and effective approaches to breast cancer control worldwide
through population-based screening mammography.
To better understand the nature of the screening programs in its member countries, IBSN has
undertaken two comprehensive assessments of member country programs in which screening
policies, funding, guidelines, and program organization were examined. IBSN also has undertaken
several quality assurance program assessments to determine the scope of quality assurance
activities for population-based screening mammography across member countries.
Three priority areas are being addressed by IBSN: 1) evaluation of screening program impact on
mortality, late-stage disease, and other surrogate measures; 2) assessment of screening program
performance parameters; and 3) evaluation of women's communication and decision-making
needs related to mammography screening.
The results from collaborative IBSN projects are published in peer-reviewed journals1,2,3 and
disseminated to all consortium members as resources for improving mammography screening
in their countries.
Additional information on the program can be found at http://appliedresearch.cancer.gov/ibsn/.
1 Yankaskas B, Klabunde C, Ancelle-Park R, Renner G, Wang H, Fracheboud J, Pou G, Bulliard J. International Breast Cancer
Screening Network. International comparison of performance measures for screening mammography: can it be done? Journal of
Medical Screening, 2004;11(4):187-193.
2 Klabunde C, Sancho-Garnier H, Taplin S, Thoresen S, Ohuchi N, Ballard-Barbash R. International Breast Cancer Screening Network.
Quality assurance in follow-up and initial treatment for screening mammography programs in 22 countries. International Journal for
Quality in Health Care, December 2002;14(6):449-461.
3 Hendrick R, Klabunde C, Grivegnee A, Pou G, Ballard-Barbash R. Technical quality control practices in mammography screening
programs in 22 countries. International Journal for Quality in Health Care, June 2002;14(3):219-226.
|
Back to Top
Improving the Quality of Cancer Care
NCI leads the Nation's efforts to develop new and better methods for cancer prevention, treatment, and symptom
management. The Institute also strives to improve the quality and delivery of cancer care throughout the world by
sharing best practices with the doctors, nurses, and other health care providers who interact directly with cancer
patients and their families.
A diagnosis of cancer presents a challenge to patients and their families - a challenge that is best met with highquality
care from a team of health care providers who have access to the latest tools and techniques of cancer care.
Should their cancer progress despite the best available care, patients will need help to reduce their pain and
suffering and to understand and face the challenges associated with the end of life. The need to improve end-of-life
care is especially urgent in developing nations, where cancer patients are often diagnosed with more advanced,
less curable disease.
The international projects described in this section highlight some of the efforts that NCI has made to improve the
quality of cancer care around the world.
Most new cancer interventions and new ways of caring for cancer patients are generated
in the industrialized world, while many of the world's cancer patients live in developing
nations. Oncologists and other health care providers in these countries have limited
opportunities to learn about the latest tools and techniques in the treatment and palliation
(symptom relief) of cancer. As a step toward addressing this dissemination problem, NCI
has cosponsored, in collaboration with the International Network for Cancer Treatment
and Research (INCTR), a set of workshops for health care providers in the Middle East
and China.
In 2003, NCI sponsored a 3-day symposium on lymphomas in Cairo, Egypt, featuring
both local and international lecturers. Concurrent with this meeting, NCI helped support
a 3-day training course for Egyptian and Palestinian oncology nurses on new developments
in cancer nursing.
Also under the framework of INCTR, NCI sponsored two pediatric oncology workshops,
one in the United Arab Emirates and one in Chongqing, China. These workshops, both
held in 2003, featured presentations on the treatment, management, and palliative care
of common childhood cancers. In addition, NCI held a workshop in Amman, Jordan for
Iraqi pediatric oncologists in 2004 to help them devise solutions for treating and caring
for pediatric patients in Iraq.
More information about INCTR can be found in Improving Cancer Communications.
NCI sponsored the first TCM Oncology Research Meeting in 2006. The conference
provided an opportunity to educate NCI staff about the application of various TCM
methods (e.g., herbs and other plant remedies, acupuncture) to prevent and treat cancer,
as well as manage symptoms. Conference organizers created a collegial environment to
engage Chinese clinical investigators and other practitioners of TCM and to facilitate
discussion about the role that NCI should take in furthering research in this area.
Presenters from China included investigators from Guang An Men Hospital of the China
Academy of TCM in Beijing, Guangzhou University of TCM in Guangzhou, and Xiyuan
Hospital of the China Academy of TCM in Beijing. Other presenters from China were
identified from a review of the relevant literature and recommendations of experts in the
field, including NCI grantees working in TCM. Invited guests included grantees in the
field of complementary and alternative medicine, NCI extramural program staff, interested
NCI intramural investigators, other National Institutes of Health program staff working
on related topics (for example, the Office of Dietary Supplements), and other TCM
investigators working in the field of oncology in the United States and elsewhere.
The Ireland-Northern Ireland-NCI Cancer Consortium has instituted numerous collaborative
programs to enhance patient outcomes in Ireland (see
Building the Capacity and Infrastructure for Cancer Research and Care for more information
about this consortium). One notable achievement of the consortium has been the
ongoing implementation of the TELESYNERGY® system. This integrated telecommunications
system of computers, microscopes, cameras, and other equipment can transmit
X-rays and other medical images or a live examination of a patient to distant sites, where
clinicians can discuss the case as if they were in the same room.
To date, five TELESYNERGY® suites have been installed on the island of Ireland under
the auspices of the All-Ireland Cancer Consortium. These suites are hosted by Belfast City
Hospital in Belfast, Northern Ireland; St. Luke's Hospital and St. James' Hospital in
Dublin, Republic of Ireland; Cork University Hospital in Cork, Republic of Ireland; and
University College Hospital Galway in Galway, Republic of Ireland.
In 2003, NCI commissioned the International Observatory on End of Life Care (IOELC)
in the United Kingdom to conduct a pilot survey of palliative and end-of-life care in the
six geographic regions represented in the Middle East Cancer Consortium (MECC) (More
information about MECC can be found below and in
Building the Capacity and Infrastructure for Cancer Research and Care). These regions include
Cyprus, Egypt, Israel, Jordan, Turkey, and the territory administered by the Palestinian
Authority. The survey's results were presented at a MECC palliative care workshop in
Cyprus in 2004.
Subsequently, NCI provided funding for IOELC to collect more information and to
prepare detailed reports on the palliative and hospice care provided in each MECC
jurisdiction. These reports were completed as part of the observatory's global analysis of
palliative and hospice care, which is intended to provide reports on palliative care services
and issues for countries and territories throughout the world in a common template to
facilitate comparative analysis.
Each IOELC report examines the current provision of palliative care, history and
development, public health context, and ethics of providing palliative care for that
country or territory. The reports for the six MECC jurisdictions are available on the
observatory's Web site at http://www.eolc-observatory.net/global_analysis/mecc.htm.
Health care providers in developing nations, including those in the Middle East, need
access to the latest tools and techniques for palliative cancer care. To help ensure broad
access to quality palliative care, NCI has worked with the Middle East Cancer
Consortium (MECC) to deliver training on palliative care to health care providers in the
Middle East and India. See above and also Building the Capacity and Infrastructure for Cancer Research and Care for more information on MECC.
In November 2005, NCI helped sponsor a second MECC palliative care workshop in
Cyprus. Researchers and clinicians from the United States, Canada, India, Pakistan, Iraq,
and the six MECC jurisdictions participated in the workshop. The topics included pain
control, psychosocial concerns, grief and bereavement, nursing issues in palliative and
home care, practical aspects of managing palliative care, and the current practice of
palliative care.
Back to Top
Improving the Quality of Life for Cancer Patients, Survivors, and Their Families
The primary focus of most doctors treating cancer patients is on producing a good clinical outcome, such as tumor
shrinkage or complete surgical resection. As important as these measures are, however, they are not necessarily the
ones that are most important to cancer patients. These "end-users" of cancer care are often more concerned with
the things that make life worth living - whether they'll see their children grow up, whether they'll be able to keep
working and supporting their families, or whether they'll be able to maintain a good or acceptable quality of life.
Many measures of a good or acceptable quality of life for cancer patients are universal, for example, adequate
pain relief or freedom from nausea. Conceptually, however, quality of life encompasses more than just the
physical aspects of well being to include the cognitive, spiritual, emotional, and social aspects of life as well.
Consequently, a "good" quality of life as defined by a cancer patient in central Africa may be very different from
that defined by a cancer patient in the suburbs of an American city.
In addition to NCI's goal of eliminating deaths due to cancer, the Institute is equally dedicated to eliminating the
suffering caused by the disease. Therefore, NCI supports and participates in research aimed at defining and
improving quality of life for cancer patients, cancer survivors, and their families throughout the world. A few
examples of this work can be found on the following pages.
In 2001, NCI created the Cancer Outcomes Measurement Working Group (COMWG)
to evaluate existing endpoint measures and instruments and to formulate alternative
strategies for identifying valid, reliable, sensitive, and feasible clinical and patient-centered
endpoint measures for use in quality of cancer care studies. The endpoint measures being
studied include health-related quality of life, patient satisfaction, and economic burden.
The COMWG is designed to help NCI and others involved in quality of cancer care
research to identify the relative strengths and weaknesses of various approaches to assessing
these endpoints. COMWG participants include researchers from the United States,
Canada, and The Netherlands.
NCI has also collaborated with the International Society for Quality of Life Research
(ISOQOL) to define quality-of-life outcome measures for people and societies around the
globe. ISOQOL is the leading organization devoted to fostering health outcomes research
and developing clinical applications to improve quality of life. NCI has participated in
and presented research at ISOQOL's annual meetings.
At the 2001 meeting in Amsterdam, The Netherlands, NCI researchers presented the
preliminary findings from the COMWG. The following year, NCI led a 2-hour panel
discussion to showcase the major findings and recommendations from the COMWG. In
addition, NCI staff helped lead a workshop on the applications of modern measurement
theory to cancer outcomes assessment and, in another workshop, discussed the application
of health-related, quality-of-life measures in clinical trials conducted by the National
Cancer Institute of Canada. More recently, NCI cosponsored and provided financial
support for the 2005 ISOQOL annual meeting in San Francisco, and NCI investigators
presented research at the conference.
In the world's industrialized societies, many people with cancer will survive their disease.
More than 25 million people throughout the world are cancer survivors and that number
grows daily. Cancer survivors have unique health needs and other concerns that must be
assessed and addressed throughout the remainder of their lives.
Assessing the state of cancer survivorship is an ongoing focus of NCI research. As part
of that research, NCI participated in a series of hearings held by the President's Cancer
Panel on cancer survivorship in the United States and Europe. At the Panel's first hearing
in the series, which was held in Lisbon, Portugal in May 2003, the key objective was to
learn about health services and survivorship activities in diverse European national health
systems that might be adapted for survivors in the United States. Additional hearings
were held in Denver, Colorado, Austin, Texas, Birmingham, Alabama, and Philadelphia,
Pennsylvania to address pediatric cancer survivorship, adolescent and young adult survivorship,
adult cancer survivorship, and older adult cancer survivorship.
 The reports that resulted from this series of hearings
are Living Beyond Cancer: Finding a New Balance
and a companion volume, Living Beyond Cancer: A
European Dialogue. They present the Panel's findings
on survivorship in the United States and Europe
and recommend action steps to alleviate the challenges
faced by cancer survivors and their families.
The reports that resulted from this series of hearings
are Living Beyond Cancer: Finding a New Balance
and a companion volume, Living Beyond Cancer: A
European Dialogue. They present the Panel's findings
on survivorship in the United States and Europe
and recommend action steps to alleviate the challenges
faced by cancer survivors and their families.
The reports address issues such as screening for disease
recurrence and the development of second cancers
and the need for ongoing medical care to deal
with the lasting effects of some treatments, such as
chronic fatigue, sexual dysfunction, or persistent pain. Survivors must also deal with the
effects that their illness may have on their employment, their families, or their ability to
fully function physically, emotionally, and socially.
Both Panel reports are available on the NCI Web site at http://deainfo.nci.nih.gov/advisory/pcp/pcp03-04rpt/Survivorship.pdf.
Back to Top
Improving Cancer Communications
NCI's communications efforts are rooted in the National Cancer Act of 1971, which directed the Institute to
"Collect, analyze, and disseminate all data useful in the prevention, diagnosis, and treatment of cancer, including
the establishment of an international cancer research data bank to collect, catalog, store, and disseminate insofar
as feasible the results of cancer research undertaken in any country for the use of any person involved in cancer
research in any country." By not limiting the range of NCI's communications activities to the United States, the
U.S. Congress showed an awareness of the complexity of cancer as a global problem and an understanding that
eliminating the suffering and death due to cancer can only be achieved by drawing on the experience, expertise,
and efforts of cancer researchers around the world.
Although the information dissemination activities dictated by the National Cancer Act were focused on cancer
researchers only, subsequent legislation widened the scope of these activities to include health professionals,
cancer patients and their families, and the general public. For example, the Public Health Service Act of 1996
called upon NCI to: provide physicians and the public with state-of-the-art information about the treatment of
various forms of cancer; identify cancer clinical trials that might benefit patients; and disseminate the results of
cancer research using information systems available to the public.
One approach NCI uses in meeting its communications requirements is to disseminate information stored and
maintained in the Physician Data Query (PDQ®) database. International cancer communications activities based
on PDQ® are described
in International Access to Information on NCI's PDQ® Database.
NCI also supports the International Network for Cancer Treatment and Research (INCTR), headquartered in
Brussels, Belgium, which helps to build capacity for cancer treatment and research in developing countries. NCI's
collaboration with INCTR is described
in greater detail
below.
The following are additional examples of NCI's international communications and outreach activities.
In 2000, the ICR Partnership was formed under the leadership of NCI and the
Congressionally Directed Medical Research Programs of the U.S. Department of Defense.
This partnership united a group of cancer funding organizations within the United States
and the United Kingdom to adopt a common language for discussing, comparing, and
presenting their cancer research portfolios. Efforts are underway to expand the membership
to include additional international partners to more fully represent the depth and
breadth of cancer research worldwide.
The ICR Partnership adopted a common coding scheme for classifying research areas -
the Common Scientific Outline (CSO) - along with an agreed upon cancer disease type
scheme, which together provided the tools needed to lay the groundwork for subsequent
portfolio analyses that will enable informed strategic planning within and among
partner organizations.
The ICR Partnership also launched the International Cancer Research Portfolio (ICRP)
web site (www.cancerportfolio.org) in 2003 to provide access to information about
cancer research supported by ICR Partnership members. This online resource allows
scientists, patient advocates, and the international cancer community to search, browse,
and sort the research portfolios of member organizations by cancer type and research
area, providing information about the funding organizations, awardee institutions, and
principal investigators along with detailed research abstracts. The international expansion
of the ICR Partnership will further enhance this resource as a step toward an integrated
global research system, allowing for improved collaboration and strategic coordination.
|
Worldwide dissemination of current, evidence-based information about cancer research, treatment, supportive care,
genetics, screening, and prevention is a high-priority activity for NCI. A number of NCI's international communications
activities are centered on information contained in the Institute's Physician Data Query (PDQ®) database.
PDQ® was launched in 1977 as an online registry of cancer clinical trials that was directly accessible by NCI staff only.
In 1982, access to PDQ® was widened when it became available through a dial-up connection to the U.S. National
Library of Medicine. In 1995, PDQ® information was made available on the World Wide Web, where it can now be
accessed through NCI's main Web site, www.cancer.gov.
PDQ® includes peer-reviewed, evidence-based information summaries about cancer treatment, supportive care,
genetics, screening, prevention, and complementary and alternative medicine. Most of these summaries are available
in two versions: one for health professionals and another for patients. PDQ® also includes a registry of more than
4,300 active and 15,000 closed cancer clinical trials from around the world. In addition, it contains directories of
persons and organizations involved in cancer care and controlled, cancer-related biomedical terminology.
In 1990, NCI's Office of International Affairs (OIA) began information dissemination projects at cancer centers in developing
countries to provide access to PDQ®, as well as to reference citations to the latest published cancer research.
NCI also developed a licensing program for PDQ® that makes information in the database available to academic, commercial,
and nonprofit organizations worldwide at no cost. Currently, NCI has international PDQ®-licensing partners in
Argentina, Chile, Germany, Japan, and the United Kingdom.
It is important to note that PDQ®-related communications activities are not solely unidirectional. NCI's Liaison Office
in Brussels, Belgium works with clinical trial groups in Europe to register their protocols in the PDQ® clinical trials
registry. Similarly, NCI's licensing partner in Japan submits Japanese clinical trial protocols for inclusion in PDQ®.
In addition to sharing the information in PDQ® with the international community, NCI also shares its experience and
expertise in developing and maintaining large cancer-related databases. In 2005, for example, NCI offered assistance
to the French Institut National du Cancer to help in the development of a comprehensive French national cancer
clinical trials registry.
|
NCI's Cancer Information Service (NCI CIS) is a founding member of the ICISG, a network
of world Cancer Information Services (CIS). The ICISG works under the auspices
of the International Union Against Cancer (UICC) to provide information and resources
regarding all aspects of cancer for those people concerned or affected by the disease.
The NCI CIS is active in ICISG's efforts to help nations, including developing nations like
Bangladesh and South Africa, develop their own CIS programs.
The ICISG is hosting a full-day workshop at the UICC's World Cancer Congress 2006
to help other nations, including developing countries, learn "How to Start a Cancer
Information Service." Topics to be covered include a basic needs assessment, scope of
services, strategic plan, staffing, and financing. The NCI CIS also developed a comprehensive
toolkit for workshop participants that provides examples of CIS training plans,
telephone call-record forms, data collection tools, training modules, and other tools that
can be used in establishing national CIS programs.
NCI provides funding and support for the International Commission on Radiological
Protection (ICRP), which was established to advance the science of radiological protection, in
particular by providing recommendations and guidance on all aspects of protection against
ionizing radiation. Recent Commission publications included a report on radiation safety
aspects of brachytherapy for prostate cancer using permanently implanted sources and another
report on protecting people against radiation exposure in the event of a radiological attack.
The ICRP is comprised of a Main Commission and four standing Committees (Radiation
Effects, Doses from Radiation Exposure, Protection in Medicine, and Application of
ICRP Recommendations), all served by a small Scientific Secretariat. Nations currently
represented on the Main Commission include Sweden, the United Kingdom, the United
States, Austria, Korea, China, Japan, Russia, Germany, and France.
|
NCI supports the International Network for Cancer Treatment and Research (INCTR), a nonprofit, non-governmental
organization founded in 1998 by the International Union Against Cancer (UICC) and the Institut Pasteur in Brussels,
Belgium. The INCTR helps build capacity for cancer research and treatment in developing nations, promotes international
collaboration directed toward cancer control between wealthier countries and those with limited resources,
and seeks unique opportunities for cancer research in developing countries.
The INCTR has established cancer research linkages in a large number of countries and regions around the world.
To aid in this effort, NCI has supported the INCTR program to provide telemedicine connections - using the National
Institutes of Health's TELESYNERGY® system - between experts and institutions in the United States and Europe with
their counterparts in developing countries.

|
| Dr. Ian Magrath |
In recent years, NCI has been a major sponsor of the INCTR's annual meetings. The 2004 meeting
in Cairo, Egypt attracted approximately 400 health care providers and researchers from more
than 40 countries, including a delegation of 25 physicians from Iraq. The Iraqi physicians were
given pediatrics textbooks, handouts, and articles relevant to workshop topics. The immediate
educational needs to improve the care of Iraqi children were identified at the meeting, such as
continuation of ongoing training, Internet and TELESYNERGY® access, and help with treatment
protocols. The 2005 annual meeting, which was held in Chennai, India, drew approximately
300 delegates from 37 countries. The 2005 meeting had sessions on topics such as emerging
technologies and palliative care.
Finally, NCI's Liaison Office in Brussels assists the INCTR with some of its education and training
activities, and Dr. Ian Magrath of NCI currently serves as the INCTR's president.
|
The Clinical Data Interchange Standards Consortium (CDISC) is an international nonprofit
organization with more than 150 members. The goal of CDISC is to develop
worldwide standards to support the electronic submission, acquisition, and exchange
of clinical trial data to improve medical research and related areas of health care. The
NCI's Enterprise Vocabulary Services (EVS) Project is working with CDISC to develop
controlled terminology for gathering and reporting clinical trial data. The NCI is also
working with CDISC's Protocol Representation Group to develop a machine-readable
and human-readable structured protocol representation standard that will support the
entire lifecycle of a clinical trial protocol. CDISC's Study Data Tabulation Model
(SDTM), which was approved by the U.S. Food and Drug Administration in 2004, has
become an internationally used standard for the tabulation and submission of clinical
research data.
Back to Top
Scientist Exchanges and Training Programs
At the heart of NCI's efforts to address the global challenge of cancer are the training and career development
programs offered at the Institute and at other biomedical research institutions around the United States. These
programs allow students and professionals at all stages of their careers to develop the skills necessary to conduct
basic, clinical, and cancer control research, as well as research in the behavioral and population sciences. It takes
a superbly trained, highly effective workforce to make groundbreaking discoveries, to translate them into new
interventions, and to put that knowledge to work to help people.
NCI's commitment to train foreign cancer researchers is manifestly evident in the cadre of international visitors
working in the Institute's laboratories. Most of these visitors are supported by NCI's visiting scientist programs,
which are described
below. Another program that attracts hundreds of international investigators every
summer is NCI's Cancer Prevention and Control Summer Academic Course, which is described below.
Additional examples of NCI programs that allow researchers from other countries to train under the mentorship of
NCI staff and grantees, as well as opportunities for U.S. researchers to go abroad, are highlighted here.
NCI's Office of Nursing Affairs (ONA) in the Institute's Center for Cancer Research
(CCR) has hosted nurses from the Ireland-Northern Ireland-NCI Cancer Consortium for
training in the roles and responsibilities of clinical research nursing. In addition, ONA
staff members have visited the King Hussein Cancer Center (KHCC) in Amman, Jordan
to help develop its clinical research programs. ONA staff presented a course on "The
Fundamentals of Clinical Research" to both nurses and doctors at the KHCC. NCI is
currently hosting Jordanian nurses at the National Institutes of Health's Clinical Center.
These nurses are being mentored in core oncology nursing training, cancer genetics, and
clinical trials.
Since 2001, NCI's Division of Cancer Treatment and Diagnosis has supported the ACRIN
Fellowship Program with the goal of training a new cadre of radiologist researchers that
is better able to develop and lead rigorous, multidisciplinary, multi-institutional clinical
trials. NCI has awarded ACRIN funds to recruit and train three new fellows a year, for
an initial period of 3 years. The NCI-funded fellows may have an interest in any subspecialty
of radiology. The program is intended to lead ultimately to the development of
independent clinical trialists. Of 13 ACRIN fellowships awarded since 2001, eight have
gone to international investigators, including one from Germany who has assumed the
role of principal investigator for the ACRIN protocol "Semi-Automated Calculation of
Volumes of Enhancing Tumor and Tumor Plus Edema from Routine Magnetic Resonance
Images in Patients with Malignant Gliomas."
NCI's longest-lasting international bilateral relationship is the 41-year-old U.S.-Japan
Cooperative Cancer Research Program, which is a joint effort of NCI and the Japan Society
for the Promotion of Science. Under this program, the United States and Japan have sponsored
more than 250 seminars and participated in more than 500 researcher exchanges.
The researcher exchange program offers junior scientists from both countries mentoring,
training, and new research experiences while conducting research projects of mutual
interest to the host laboratories. Research workshops have also been held on numerous
topics, including "Cancer Immunosurveillance" and "Cancer Vaccine Clinical Trials."
|
Persuasive evidence of NCI's commitment to train foreign cancer researchers can be found in the cadre of international
visitors working in NCI laboratories. In fiscal year 2005, over 1,000 of these visitors, representing 74 countries,
contributed to intramural projects being conducted at NCI's Center for Cancer Research (CCR) while preparing
themselves for their own careers as cancer researchers. The major contribution these training activities make
toward the worldwide effort to eliminate the suffering and death due to cancer is evidenced by the fact that many of
the current leaders in cancer research around the globe spent time at NCI earlier in their careers. Most of these
international visitors to NCI's laboratories have been supported by the NIH Visiting Program.
In addition to these training activities at NCI facilities, NCI's Office of International Affairs (OIA) partially supports
the training of scientists from developing countries in non-NCI laboratories across the United States as well as in
laboratories located in other countries. In fiscal year 2005, OIA supported scientists from Argentina, Cameroon, China,
Egypt, Poland, and Venezuela who were trained at cancer institutes, hospitals, and universities located in the United
States. Also that year, OIA sponsored trainees from Afghanistan, Belarus, Bolivia, Brazil, Iran, Jordan, Ukraine, and
the West Bank who were trained in laboratories located in France, Israel, India, and the United Kingdom.
More information about the NIH Visiting Program can be found at
http://dis.ors.od.nih.gov/aboutnihvp.htm.
|
In 1997, NCI convened the first scientific symposium focused on AIDS-associated
malignancies. The scientific community soon identified the need for greater cooperation
and collaboration to address the problem of AIDS-associated cancer and this annual
symposium became an international event. As of 2005, NCI has sponsored nine of
these symposia.
The symposium serves as a forum for the presentation of basic, epidemiologic, and clinical
aspects of research on malignancies in human immunodeficiency virus-1 (HIV-1)-infected
and other immunosuppressed individuals.
Participants include clinical and laboratory investigators, postdoctoral fellows, and students.
The symposium also draws physicians and health care workers who are interested
in, or participating in, malignancy research in AIDS, other immunodeficiencies, and
tumor virology from the United States, Europe, Asia, Africa, and South America. The
symposium represents the only meeting of its kind to describe the current state-of-the-science,
identify challenges and opportunities, and stimulate research cooperation across the
globe in the field of AIDS-associated malignancies.
In 1999, a historic memorandum of understanding was signed that established the
Ireland-Northern Ireland-NCI Cancer Consortium. The primary goal of this consortium
is to enhance the infrastructure for cancer research and cancer care across all of Ireland.
In addition to facilitating interactions among the three research communities represented,
the Consortium has developed a number of joint programs to address the entire continuum
of cancer (see related article in
Building the Capacity and Infrastructure for Cancer Research and Care). One of these programs supports scholar
exchanges that have, to date, involved three Irish scholars who were given 3-year fellowships
in epidemiology. One year of these fellowships was spent working with NCI's
Surveillance, Epidemiology, and End Results (SEER) Program in Bethesda, Maryland.
NCI has also hosted more than a dozen Irish nurses, who trained in oncology nursing
and clinical trials for 3 months at the National Institutes of Health's Clinical Center in
Bethesda, Maryland (see above).
Additional evidence of this training commitment is demonstrated by the fact that over
80 researchers and health care providers from Ireland have participated in NCI's summer
academic course on the principles and practice of cancer prevention and control (see
related article below).
|
Each summer, visitors from a number of foreign countries participate in the Principles and Practice of Cancer
Prevention and Control Course offered by NCI's Division of Cancer Prevention (DCP). In 2005, course participants
included scientists and health professionals from China, Ghana, India, Lithuania, Russia, and Turkey.
The course focuses on concepts, methods, issues, and applications related to the field of cancer prevention and
control. Participants have an opportunity to gain a broad-based perspective of these subjects, including resources,
data, methods, and theories. Instilled lifetime learning skills include critical appraisal of the literature and bibliographic
search concepts and techniques.
Given the large number of international participants, most of whom are accomplished scientists, one day is set
aside to provide an opportunity for these participants to share their expertise with other course attendees through
presentations on issues related to cancer prevention in their home countries.
The Principles and Practice of Cancer Prevention and Control Course is part of DCP's NCI Summer Curriculum in
Cancer Prevention, which also includes a Molecular Cancer Prevention Course and an Annual Advances in Cancer
Prevention Lecture.
More information about DCP's NCI Summer Curriculum in Cancer Prevention and the Principles and Practice of
Cancer Prevention and Control Course can be found at http://www3.cancer.gov/prevention/pob/courses/index.html.
|
Back to Top
Building the Capacity and Infrastructure for Cancer Research and Care
To achieve our goal of eliminating the suffering and death due to cancer, we must build and sustain strong research
environments, support structures, collaborations, and communication channels that will enable us to quickly pursue
new ideas, translate discoveries into innovative interventions, and disseminate evidence-based treatments and
practices worldwide. We must nurture and maintain a cadre of scientists and health care professionals in a variety
of disciplines, and we must strive to eliminate barriers to information, research opportunities, and adequate care.
Therefore, NCI seeks to share its expertise and to facilitate capacity building and infrastructure development to
strengthen cancer research and improve the quality of cancer care around the world. These efforts are especially
important in nations that have limited resources.
The following are several examples of NCI's efforts aimed at increasing the capacity and building the
infrastructure for high-quality cancer research and cancer care worldwide.
In 1999, a historic memorandum of understanding was signed that established the
Ireland-Northern Ireland-NCI Cancer Consortium. The primary goal of this consortium
is to enhance the infrastructure for cancer research and cancer care across all of Ireland.
In addition to facilitating interactions among the three represented research communities,
the consortium has developed a number of joint programs covering the entire continuum
of cancer.
The range of the consortium's activities can be gauged by the focus of its working groups:
Scholar Exchange, Clinical Trials, Cancer Registries, Nursing, Information Technology/
Telecommunications, and Prevention. Several of these activities are discussed elsewhere in
this portfolio, but one program particularly worth mentioning here is the effort to provide
fellowships and training for Irish scientists and nurses at NCI's headquarters in Bethesda,
Maryland (see
Scientist Exchanges and Training Programs).
The Consortium's Prevention Working Group has been particularly active. In addition to
participating in workshops in Ireland on topics such as "Cancer Prevention and Tobacco
Control" and "Obesity and Cancer," the working group has dedicated itself to building
an Ireland-wide community of prevention-oriented scientists and cancer caregivers.

|
| Dr. Samir Khleif |
In September 2002, the King Hussein Cancer Center (KHCC) in Amman, Jordan forged
a cooperative agreement with NCI for the purpose of enhancing medical sciences and
improving cancer patient care in Jordan and the entire Middle East region. In support of
the agreement, Dr. Samir Khleif, a clinical oncologist at NCI, was named as KHCC
Director General.
One of the notable achievements of this agreement occurred in 2004, when NCI Director
Andrew von Eschenbach and U.S. Department of Health and Human Services Secretary
Tommy Thompson visited KHCC to launch its TELESYNERGY® suite, which will help
foster collaboration between cancer specialists, facilitate professional education and training,
and permit consultation in cancer research protocols and patient care throughout
Jordan and the Middle East, at selected sites in the United States, and throughout Ireland
(see also
A telemedicine technology program to share expert advice and diagnostic input
between the NCI and Ireland to improve patient outcomes).
|
NCI supported the 5th International Conference on Cancer in Africa, which was held in Dakar, Senegal in
November 2005.
The conference, hosted by the African Organization for Research and Training in Cancer, was designed to develop
a research agenda for dealing with the increasing crisis of cancer in Africa. This research agenda will focus on
screening, prevention, and management of high-incidence cancers, such as breast, cervical, and prostate cancer.
The conference sought to establish global partnerships among oncology care givers and to address health care
disparities, with a focus on early detection and cancer prevention.
The problem of tobacco-related cancers in Africa was one of the issues discussed at the conference. Participants discussed
ways to combat the growing incidence of these cancers as the tobacco industry turns more to the developing
world to find markets for its products.
Also discussed was the problem of how to pay for cancer care for patients who have no resources or health insurance.
Funding adequate cancer care in such resource-poor environments presents monumental challenges. Early
detection and cancer prevention were recommended as ways to minimize both the costs and the risks posed by
cancer. In Africa, most cancer patients do not see health care providers until their disease has progressed to an
advanced stage. Calls were made to encourage women to conduct breast self-examinations and to get Pap smears
at least once every 2 years. Cervical cancer is the most prevalent cancer among African women, despite being the
most preventable of all gynecologic cancers.
Finally, the importance of improving the availability and use of palliative care to relieve cancer symptoms and the
adverse effects of treatment among African cancer patients was stressed.
|
NCI, along with other institutes of the National Institutes of Health, cosponsors awards
to establish and maintain Centers for AIDS Research (CFARs) at U.S. institutions that
receive substantial AIDS funding from multiple NIH sources. The mission of each CFAR
is to support a multidisciplinary environment that encourages collaboration across basic
and clinical studies and promotes research in the prevention, detection, and treatment of
HIV infection, AIDS, and AIDS-associated diseases, including cancer. Currently, there are
20 CFARs at U.S. research institutions.
One way in which the CFARs seek to accomplish their mission involves strengthening
the capacity for HIV/AIDS research in developing countries. The Case Western Reserve
University CFAR's activities in the African nation of Uganda serve as an example of these
efforts. Building on its longstanding relationship with Makere University in Kampala,
Uganda, the Case Western CFAR worked to establish the Uganda Laboratory Core in
1997. This laboratory facility created the capacity in Uganda to perform state-of-the-art
immunology and virology research and to provide laboratory services for health care
professionals throughout central Africa. It also provided opportunities to extend the scientific
activities of the Uganda-Case Western Reserve University Research Collaboration
to include studies in AIDS-related malignancies.
In the last four years, the rapid growth and evolution of national cancer control planning
has led to highly visible and successful initiatives by NCI's Cancer Information Service
(NCI CIS) that are designed to improve public health in the United States. In these efforts,
the NCI CIS joined with a U.S.-based group of experts, including the American Cancer
Society, the Centers for Disease Control and Prevention, and the Intercultural Cancer
Council to develop the Comprehensive Cancer Control Leadership Institutes (CCCLIs).
Domestically, CCCLIs provide an opportunity for highly skilled, influential individuals in
the United States to take action together to support implementation efforts for a comprehensive
cancer control approach in their respective states.
With the success of the CCCLIs in the United States, the CIS and its partners began to
address the need for global comprehensive cancer control planning in 2004. The main role
for the CIS in these international efforts is capacity building. In September 2004, the CIS
helped form a new international comprehensive cancer control group that is investigating
the feasibility and the interest in an international version of the CCCLIs. The group consists
of federal and non-federal partners including experts from NCI, the U.S. Centers for
Disease Control and Prevention, the American Cancer Society, and the International Union
Against Cancer (UICC). In 2005, the NCI CIS supported Peruvian cancer leaders in developing
a cancer control plan for that nation. The first pilot of an international CCCLI
workshop took place in Mexico City in June 2006, with participation from Brazil, Mexico,
Peru, and Uruguay. In addition, two workshops on cancer control planning are being held
at the UICC's World Cancer Congress 2006 meeting in Washington, D.C.
|
 The Middle East Cancer Consortium (MECC), which was established in 1996, is a unique partnership involving the
United States and the Ministries of Health of Cyprus, Egypt, Israel, Jordan, the Palestinian Authority (Gaza and the
West Bank), and Turkey. One of the consortium's principal initiatives is its Cancer Registry Project, which supports
the development of high-quality, population-based cancer registries in all MECC jurisdictions.
The Middle East Cancer Consortium (MECC), which was established in 1996, is a unique partnership involving the
United States and the Ministries of Health of Cyprus, Egypt, Israel, Jordan, the Palestinian Authority (Gaza and the
West Bank), and Turkey. One of the consortium's principal initiatives is its Cancer Registry Project, which supports
the development of high-quality, population-based cancer registries in all MECC jurisdictions.
To facilitate registry training, MECC established an education program and developed a "Manual of Standards for
Cancer Registration." The manual, which is now in its fourth edition (2005), defines requirements for data collection
and coding that are to be followed by all MECC registries. In addition, staff from NCI's Surveillance, Epidemiology,
and End Results (SEER) Program have provided assistance with training, technical support, and quality control.
The first report1 based on MECC-affiliated registry data was published in the European Journal of Cancer Prevention
in 2003. This report contained the first-ever comparison of cancer incidence rates in Israel and one of its Arab
neighbors, Jordan. The report had Israeli, Jordanian, and American authors, including three NCI scientists.
The first monograph from the MECC Cancer Registry Project was
released in 2006. This monograph, entitled Cancer Incidence in Four
Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East
Cancer Consortium (MECC) Compared with U.S. SEER, provides information
about cancer incidence for populations in Cyprus, Egypt (Gharbiah
Region), Israel (Jews and Arabs), and Jordan for the period 1996-2001 in
comparison with data reported by SEER for the United States. This monograph
can be accessed on the Internet at http://seer.cancer.gov/publications/mecc/mecc_monograph.pdf. Alternatively, a print copy can be
ordered from SEER (http://seer.cancer.gov/publications).
1 Freedman L, Barchana M, Al-Kayed S, Qasem M, Young J, Edwards B, Ries L, Roffers S, Harford J, Silberman M. A comparison of population-based cancer incidence
rates in Israel and Jordan. European Journal of Cancer Prevention 12(5):359-365, 2003.
|
Back to Top
|