 |
|
 |

Genome Surveys Reveal Complexity of Brain Cancers
The most comprehensive studies to date of the molecular changes underlying brain cancer were published last week. The information significantly expands current knowledge about the genetic networks involved in this deadly disease and points to potential therapeutic strategies.
The Cancer Genome Atlas 1 (TCGA) Research Network analyzed 206 glioblastoma (GBM) brain tumors using an integrated approach based on multiple types of genetic data and clinical information. Reporting their findings online in Nature 2, the researchers identified gene mutations not previously recognized in the disease and a core set of molecular pathways that are commonly deregulated in the cancer, frequently together.
An unexpected finding that could be translated into the clinic within the next few years, the research team said, was the discovery of a potential mechanism of resistance to temozolomide 3, a chemotherapy drug for brain cancer.
Read more 4 


Palliative Care Consultation Lowers Hospitalization Costs
Adding palliative care consultation to standard care for patients who have a serious illness can reduce hospitalization costs significantly, according to researchers from The Palliative Care Leadership Centers' Outcomes Group. The group's analysis appeared yesterday in the Archives of Internal Medicine and showed an adjusted net savings of $279 per day for palliative care patients who were discharged alive and a savings of $374 per day for patients who received palliative care consultation but died during their hospital stay. Such consultations outline a patient's treatment priorities and can help avoid unnecessary tests or treatment that might otherwise be used to prolong life at any cost.
This retrospective, nonrandomized study focused on patient records from 2002 to 2004 at eight hospitals around the United States, representing low-, medium-, and high-cost markets. All hospitals employed experienced palliative care consultation teams, and the use of palliative care and related costs were identified by billing codes. The analysis matched 2,630 palliative care patients with 18,427 usual-care patients who were discharged alive, and 2,278 palliative care patients who died in the hospital were matched with 2,124 usual-care patients who died in the hospital.
Read more 5 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Genome Surveys Reveal Complexity of Brain Cancers
The most comprehensive studies to date of the molecular changes underlying brain cancer were published last week. The information significantly expands current knowledge about the genetic networks involved in this deadly disease and points to potential therapeutic strategies.
The Cancer Genome Atlas 1 (TCGA) Research Network analyzed 206 glioblastoma (GBM) brain tumors using an integrated approach based on multiple types of genetic data and clinical information. Reporting their findings online in Nature 2, the researchers identified gene mutations not previously recognized in the disease and a core set of molecular pathways that are commonly deregulated in the cancer, frequently together.
An unexpected finding that could be translated into the clinic within the next few years, the research team said, was the discovery of a potential mechanism of resistance to temozolomide 3, a chemotherapy drug for brain cancer.
"This study demonstrates that an unbiased, comprehensive global search for alterations in a large collection of tumors can definitely yield valuable insights that were not anticipated," said Dr. Lynda Chin of Dana-Farber Cancer Institute, who co-chaired the TCGA committee that wrote the Nature paper with her colleague, Dr. Matthew Meyerson.
These are the first results from the TCGA pilot project, 6 a collaborative effort funded by NCI and the National Human Genome Research Institute to establish the feasibility of using integrated genomic strategies to characterize the molecular alterations in cancer. NCI Deputy Director Dr. Anna Barker co-leads the research program, which includes investigators from 18 institutions and organizations.
The current project involved characterizing tumors for alterations in DNA copy number, gene expression, and DNA methylation status - an epigenetic change that regulates gene activity. The researchers also sequenced 601 genes in 91 of the GBM tumors.
The study highlights three genes involved in the disease - ERBB2, NF1, and TP53. In addition, three pathways - RB, p53, and RTK/RAS/PI3K - were interconnected and deregulated in most, if not all, of the GBM tumors analyzed. Combination therapies directed against these pathways may be an effective strategy for some patients, the researchers suggest.
"In study after study, we see that cancer-causing genes fall into certain pathways, and that these pathways can be altered in many different ways," said Dr. Meyerson. "And it is only by getting a comprehensive picture of these changes that you begin to understand the genetic landscape of these tumors."
The temozolomide finding involved the gene MGMT, which, when methylated (that is, inactivated), can predict sensitivity to the drug. The data suggest that in some patients with a methylated MGMT gene temozolomide could lead to resistance as well as to mutations in genes that are essential for repairing DNA.
"If confirmed, the finding would immediately give us a handle on how to design combination therapies to limit the development of secondary cancers and how to better use temozolomide," said Dr. Chin. "This finding is a good example of what you want to achieve with a cancer genome project," she added, because the insight was both unexpected and emerged only after diverse types of information were analyzed together.
The second study, co-led by Drs. Bert Vogelstein, Kenneth Kinzler, and Victor Velculescu of the Johns Hopkins Kimmel Cancer Center, analyzed nearly every human gene in 22 glioblastoma tumors. Their report, published online in Science 7, is accompanied by an analysis of 24 pancreatic cancers 8, another disease that, like brain cancer, needs new therapies and methods of early detection. The team has previously published genomic analyses 9 of 11 breast and
11 colon tumors.
As in the TCGA study, the Johns Hopkins group integrated different types of genomic data and identified altered genes and pathways. A gene called IDH1, which was not previously associated with GBM, was altered in 18 of the 149 GBM tumors they analyzed.
The TCGA report and the complementary study by the Johns Hopkins investigators suggest that it will be possible to define cancer subtypes on the basis of activated pathways by developing multi-dimensional data on robust numbers of patient samples, noted Dr. Barker. "This level of understanding offers real hope for defining evidence-based therapies and diagnostics," she said.
TCGA is currently characterizing additional GBM cases and will
obtain even more cases. "These results are not the final statement on this disease, but they do suggest that what we learn will lead us in new directions for patient care," said Dr. Daniela Gerhard of NCI's Office of Cancer Genomics.
—Edward R. Winstead
|
 |
 |
Palliative Care Consultation Lowers Hospitalization Costs
Adding palliative care consultation to standard care for patients who have a serious illness can reduce hospitalization costs significantly, according to researchers from The Palliative Care Leadership Centers' Outcomes Group. The group's analysis appeared yesterday in the Archives of Internal Medicine and showed an adjusted net savings of $279 per day for palliative care patients who were discharged alive and a savings of $374 per day for patients who received palliative care consultation but died during their hospital stay. Such consultations outline a patient's treatment priorities and can help avoid unnecessary tests or treatment that might otherwise be used to prolong life at any cost.
This retrospective, nonrandomized study focused on patient records from 2002 to 2004 at eight hospitals around the United States, representing low-, medium-, and high-cost markets. All hospitals employed experienced palliative care consultation teams, and the use of palliative care and related costs were identified by billing codes. The analysis matched 2,630 palliative care patients with 18,427 usual-care patients who were discharged alive, and 2,278 palliative care patients who died in the hospital were matched with 2,124 usual-care patients who died in the hospital.
The researchers found that palliative care consultation saved $1,696 per patient admitted if the patient survived, mostly attributed to lower laboratory costs ($424 per admission) and lower ICU costs ($5,178 per admission). The savings per admission for palliative care patients who died in the hospital were $4,908. Length of stay in the hospital ranged from 7 to 30 days.
"Our data suggest that palliative care consultation fundamentally shifts the course of care…," the authors write, "and in doing so, significantly reduces costs. This shift is likely accomplished by establishing clear treatment goals, reviewing current treatments to establish their concordance with these goals, and recommending and legitimizing discontinuation of treatments or tests that do not meet established goals."
More Treatments for Cancer-Related Fatigue Needed
Cancer-related fatigue commonly affects patients both during and after treatment. In a systematic review of pharmacologic treatments for cancer-related fatigue, investigators from St. George's University of London found that the psychostimulant methylphenidate provided a small but significant reduction in fatigue compared with placebo in two studies of 264 patients. In addition, the hematopoietic growth factor erythropoietin provided a clinically significant reduction in fatigue in 10 studies of 2,226 anemic patients undergoing chemotherapy, although the doses given and duration of treatment varied widely between trials. The study appeared in the August 20 Journal of the National Cancer Institute 10.
The investigators searched the Cochrane, Medline, and EMBASE databases of medical literature, and performed additional searches of selected journals and reference lists from identified articles. They found 27 randomized controlled trials, with a total of 6,746 participants, that tested a drug against placebo or standard care, aimed to improve quality of life, and robustly measured fatigue.
The review also included trials that tested darbepoetin, the antidepressant paroxetine, and progestational steroids. None of these drugs had a statistically significant effect on fatigue, though there was a borderline significant reduction with the use of darbepoetin in anemic patients.
Overall, the effects of both methylphenidate and erythropoietin were small, and both drugs have drawbacks, explain the authors. Methylphenidate is potentially addictive, and research has not identified which patients are most likely to benefit from treatment. Recent studies have raised safety concerns 11 about erythropoietin.
"Future research into cancer-related fatigue…should not focus simply on the role of drugs," conclude the authors. Exercise and psychological techniques such as cognitive behavioral therapy may potentially help with fatigue, but need to be tested in adequately designed clinical trials.
In addition, explain the authors, "Many potential mechanisms and contributing factors could cause or increase the level of cancer-related fatigue…if mechanisms that produce fatigue could be identified, it may also be possible to design more targeted drug therapies or other interventions."
Evidence-Based Standards Developed for Pain Control
An expert panel of nine researchers from academic and community settings has published key standards and recommendations for cancer pain management. Development of the standards, published in the August 10 Journal of Clinical Oncology 12, involved a systematic literature review and deliberations by the expert panel, which rated each recommendation on validity and feasibility.
For general pain management, Dr. Sydney M. Dy of The Johns Hopkins University and his colleagues, recommend routine screening, descriptive pain assessment for etiology and functional impairment, routine pain education, and follow-up of pain management. For most metastatic bone pain, single-fraction radiation treatment is as effective as multiple treatments, they say, and should be offered whenever clinically possible. For back pain and spinal compression, the authors recommend quick treatment with corticosteroids, imaging with whole-spine MRI or myelography, and starting definitive treatment - such as radiotherapy or surgical decompression - within 24 hours.
The recommendations also address the common side effects that often arise in patients receiving chronic or long-acting opioids for pain, such as morphine, oxycodone, and fentanyl. These patients, the authors write, should receive bowel regimens to counter constipation; their dosage should be carefully monitored when they switch treatment settings; and they should receive breakthrough pain medications as needed.
"Pain is one of the most common...symptoms in cancer," the authors write. "These standards provide an initial framework for high-quality evidence-based management of general cancer pain and pain syndromes."
Math Model Projects Health and Economic Effects of HPV Vaccine
The effect of HPV vaccination on cervical cancer outcomes in the United States won't become evident for many years. In the meantime, to address questions about who should receive the vaccine, researchers from the Harvard School of Public Health have used a mathematical model to project the cost-effectiveness of vaccinating 12-year-old girls, as well as vaccinating older girls and women up to the age of 26. Their analysis appeared August 21 in the New England Journal of Medicine 13.
The authors' calculations were built on a model of male and female sexual behavior over time, as well as the carcinogenic effects of the virus in cervical tissue. It accounted for both forms of the vaccine that are currently used in clinical practice, the effect of waning immunity after vaccination with and without boosters, and possible protection against strains other than HPV type 16 and 18 that aren't part of the current vaccine formula.
Assuming lifelong immunity, their calculation showed that routine vaccination of 12-year-old girls, in addition to routine cervical screening, costs $43,600 per quality-adjusted life year (QALY) gained, above the cost of screening alone. For girls aged 13 to 18 years, the cost was $97,300 per QALY. Extending vaccination to women aged 21 cost $120,400 per QALY, and up to 26 years of age, the cost was $152,700 per QALY.
In projections where the vaccine protected against HPV types 6 and 11, which cause genital warts, the costs were reduced by 13 to 20 percent. (The reductions diminished as age increased.) However, factors that increased the cost per QALY for all age groups included immunity lasting only 10 years, thus requiring a booster, and a scenario in which 5 percent of the female population aged 26 and younger did not get screened or vaccinated. Frequency of screening and testing protocols also affected the cost, which could go as high as $200,000 per QALY.
The cost-effectiveness of HPV vaccination in the United States will depend on the duration of vaccine immunity and "will likely be optimized by achieving universal coverage in young adolescent girls and targeting initial catch-up efforts to girls and women younger than 21 years of age," the researchers conclude.
Phase III Trial of Immunotherapy for Prostate Cancer Stopped
A phase III clinical trial testing a prostate cancer immunotherapy treatment in men with advanced, hormone-refractory prostate cancer (HRPC) has been halted because of a greater number of deaths in patients receiving the investigational treatment. The company that developed the treatment, Cell Genesys, announced the trial's termination following a recommendation made by its Independent Data Monitoring Committee (IDMC).
Men in the trial, dubbed VITAL-2 14, were randomly assigned to either the combination of an investigational immunotherapy treatment called GVAX and the chemotherapy drug docetaxel 15 or the combination of docetaxel and the corticosteroid prednisone. More than 400 patients were enrolled in the trial, and at the time of its analysis, 114 deaths had occurred, 67 in the GVAX arm and 47 in the docetaxel/prednisone arm.
"A specific cause for the imbalance in deaths has not been identified," Cell Genesys said in a statement. "The Company plans to fully analyze the clinical data from these patients to attempt to understand the potential cause for the higher rate of deaths observed in the GVAX arm."
A second phase III trial of GVAX, called VITAL-1, is evaluating GVAX as a monotherapy in earlier stage HRPC patients with metastatic disease. Its IDMC has not recommended halting the trial. The company has asked the committee, however, to conduct a "futility analysis" of VITAL-1 to determine whether the trial is likely to achieve its primary endpoint of improved survival in the GVAX arm.
|
 |
 |

Guest Update by Dr. Ann O'Mara
Expanding the "Power of Palliation" Through Research
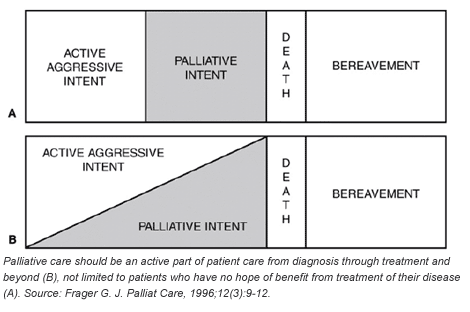
 Many people assume that palliative care is for the elderly or those on the brink of death, people who have so little time left that they should not be made to suffer discomfort from an illness or receive any more treatment. In truth, palliative care aims to minimize the harmful effects of a person's illness as well as the harmful effects of treatments from the point of diagnosis throughout the treatment process and beyond. These harmful effects can be physical (pain, dry mouth, heart weakness), mental (loss of memory, mental acuity), social (living without caretakers, lack of insurance), and emotional (depression, anxiety).
Many people assume that palliative care is for the elderly or those on the brink of death, people who have so little time left that they should not be made to suffer discomfort from an illness or receive any more treatment. In truth, palliative care aims to minimize the harmful effects of a person's illness as well as the harmful effects of treatments from the point of diagnosis throughout the treatment process and beyond. These harmful effects can be physical (pain, dry mouth, heart weakness), mental (loss of memory, mental acuity), social (living without caretakers, lack of insurance), and emotional (depression, anxiety).
 |
 |
Resources on Palliative Care |
 |
|
While advances in treatment are improving survival and reshaping cancer as a chronic disease, palliative care has become more important for cancer patients throughout the spectrum of their disease. For example, in 2004, a 28-year-old man came to NIH after being diagnosed with a rare metastatic tumor in his abdomen. After surgery, chemotherapy, and a stem cell transplant, his cancer recurred the following year. Today he is enrolled in a treatment clinical trial and he uses a variety of pharmacologic and behavioral therapies for the symptoms associated with his disease and its treatment, including pain and anxiety. He lives an otherwise normal life and has successfully started a career as an artist. Without a holistic approach to his cancer care, this young man's story might be very different.
Still, a host of research questions about palliative care remain. For instance, there is no effective treatment for the nerve damage caused by chemotherapy or for EGFR inhibitor-induced skin rashes and infections. Patients and families remain reluctant to report treatment- and disease-related symptoms. Methods for health care providers to document treatment-related toxicities are inefficient or inadequate. Clinicians continue to have trouble relating "bad news" to patients and may delay referring patients to hospice and other supportive care services.
In response to these and other
related issues, NCI is collaborating with other NIH institutes to stimulate research 16 in several categories of palliative care that affect multiple diseases. In 2005, NCI partnered with the National Institute of Nursing Research and the Office of Research on Women's Health to fund 16 research projects 17 aimed at improving the delivery of treatments to prevent or lessen the adverse physical and psychosocial consequences associated with cancer and its treatment. Many of the investigators are entering into the fourth year of their projects and some findings have been published, including studies on barriers to assessing and managing pain and what African American patients want from their physicians.
NCI also takes part in consortia and working groups created by NIH, such as the NIH Pain Consortium, Sleep Disorders Consortium, Fertility Preservation Work Group, and Medical Rehabilitation Advisory Board, to maximize research efforts. At NCI, the Symptom Management/Quality of Life Steering Committee 18 focuses on guiding and initiating cancer-related palliative care studies. The research bases of NCI's Community Clinical Oncology Program 19 also conduct clinical trials on palliative care within their network.
Susan Lowell Butler, a 13-year survivor of breast and ovarian cancer, can summarize the value of palliative care throughout cancer treatment. "I believe that palliative care should be an intrinsic part of the care plan for every cancer patient, from the time of diagnosis until the ultimate outcome of care, including long-term survivorship. The relief of pain and other side effects of the disease and its treatments are essential for a good quality of life, and people with cancer and those loved ones who care for them rely on the health care team to make them aware of the power of palliation."
Dr. Ann O'Mara
Head of Palliative Care Research
Community Oncology and Prevention Trials Research Group
NCI Division of Cancer Prevention
|
 |
 |

Gene Mutations Identified as Cause of Neuroblastoma
For more than a decade, parents of children with neuroblastoma have been asking Dr. John Maris what causes the cancer, and until now he has had no answer. But new research led by his team at the Children's Hospital of Philadelphia (CHOP) is starting to provide clues that could lead to a genetic test and a clinical trial as early as next year.
The researchers have identified germline mutations 20 in the anaplastic lymphoma kinase (ALK) gene in the vast majority of families with the inherited form of the disease. The discovery has generated interest in the field because ALK is a known cancer gene (oncogene) and drugs against this target are in development. The ALK protein - a receptor tyrosine kinase - helps regulate cell growth and may be abnormally activated in cancer.
"This is an incredibly exciting discovery," said Dr. Susan Cohn, an expert on neuroblastoma at the University of Chicago, who was not involved in the study. "If we're lucky, the discovery will dramatically impact the way we approach patients with neuroblastoma in the future."
While familial cases of neuroblastoma account for only 1 percent of all cases, ALK mutations can also occur as tumors grow and spread in the more common non-hereditary forms. The researchers found ALK mutations in 12 percent of high-risk neuroblastoma tumor samples, and the gene is amplified (present in multiple copies) in up to an additional 5 percent of tumors.
Neuroblastoma arises in children in the developing cells of the sympathetic nervous 21 system, and it often appears as a tumor in the chest or abdomen. Half of children with the disease have the high-risk form, which has long-term survival rates of only 40 percent at best and often resists standard therapies.
Reporting their findings online in Nature 22 last month, the researchers concluded that ALK mutations appear to drive the disease in a subset of neuroblastoma patients. Even before the initial findings were presented at a scientific meeting last spring, other laboratories had implicated ALK mutations in the disease.
By early 2009, the researchers expect to have a genetic test available. The test could identify family members of patients with neuroblastoma who harbor ALK mutations. These individuals could be carefully monitored using noninvasive techniques such as ultrasound or a urine test. If the ALK protein turns out to be an important therapeutic target, testing could be applied broadly to guide treatment.
Dr. Maris and his coauthor, Dr. Yael P. Mossé, also at CHOP, are in discussions with a pharmaceutical company about opening a trial of an ALK inhibitor for children with neuroblastoma next year.
The ALK gene was discovered in 1994 in patients with a large-cell lymphoma. It has since been shown to play a role in multiple cancers, including lung and esophageal. In these diseases, ALK merges with other genes in "translocations" that drive cell growth by activating the ALK kinase.
To demonstrate a role for ALK in inherited neuroblastoma, the researchers scanned the DNA of 10 families with two or more affected members. Three inherited mutations in ALK tracked with the disease in eight separate families.
The researchers then analyzed nearly 200 high-risk neuroblastoma samples and found spontaneous, or non-inherited, mutations in 12 percent. An effort to characterize the full spectrum of ALK mutations and when the mutations occur is underway.
"There appear to be some mutations where all individuals who carry them will manifest the disease and other mutations that are less likely to cause the disease," said Dr. Mossé. It may be that a second genetic "hit," such as the amplification of ALK, is required to cause the disease in most patients, she noted.
An interesting finding of the study was that experimentally inhibiting ALK signaling reduced the proliferation of neuroblastoma cells, even in cells with no known ALK mutations. This suggests that targeting ALK may benefit patients with normal ALK genes, but the researchers caution that clinical trials are needed to answer these questions. This is likely to happen soon.
"Translating this discovery from the Maris laboratory into the clinic will be a high priority for neuroblastoma researchers," said Dr. Malcolm Smith of NCI's Cancer Therapy Evaluation Program 23.
Dr. Smith, along with Dr. Maris, leads the Neuroblastoma TARGET 24 Initiative, which uses genomic strategies to identify and validate therapeutic targets for the disease. The group is already sequencing the ALK gene in additional neuroblastoma cases and other genes as well.
In a genome-wide association study 25 published last May, Dr. Maris' group identified a region of chromosome 6 that may contain genetic variants associated with aggressive neuroblastoma. This study and the current one were completely separate, but each produced novel insights into the genetics of neuroblastoma, noted Dr. Maris.
"We know a whole lot more about what causes the disease today than we did a year ago," he said.
—Edward R. Winstead
|
 |
 |

Targeting Occult Cancer Cells in High-risk Prostate Cancer Patients
Name of the Trial
Phase III Randomized Study of Radical Prostatectomy with Versus without Neoadjuvant Chemohormonal Therapy Comprising Docetaxel and Androgen-deprivation Therapy with Leuprolide Acetate or Goserelin in Patients with High-risk, Clinically Localized Prostate Cancer (CALGB-90203). See the protocol summary at http://www.cancer.gov/clinicaltrials/CALGB-90203.
Principal Investigators
Dr. James Eastham, Cancer and Leukemia Group B; Dr. Martin Sanda, Eastern Cooperative Oncology Group; Dr. Martin Gleave, NCIC-Clinical Trials Group
Why This Trial Is Important
Prostate cancer is classified as localized when there is no evidence it has spread (metastasized 26) to nearby tissue or lymph nodes 27. However, patients treated with surgery to remove the prostate and some surrounding tissue (radical prostatectomy 28) often experience recurrence of their disease due to the presence of undetectable (occult) cancer cells.
Doctors want to improve their ability to identify prostate cancer patients who fall into this high-risk group (i.e., men who have less than a 60 percent chance of remaining recurrence-free 5 years after treatment). One way to identify these patients takes into account factors such as tumor stage, higher levels of prostate-specific antigen 29 (PSA), and other clinical signs, such as a higher Gleason score 30. However, said Dr. Eastham, "Once we identify them, there is no accepted treatment strategy for high-risk patients."
Some high-risk patients elect treatment before surgery (neoadjuvant therapy 31) in hopes of improving their long-term outcome. If their cancer's growth is dependent upon male hormones (androgens), anti-androgen therapy before surgery can reduce the extent of their disease. If their cancer is androgen-independent 32, systemic chemotherapy 33 with a taxane 34 drug has been shown to improve long-term survival.
In this trial, patients classified as high risk will be randomly assigned to undergo a course of neoadjuvant therapy or proceed directly to surgery. The neoadjuvant therapy will involve up to 18 weeks of docetaxel 35 chemotherapy combined with hormone-depriving therapy using one of two drugs, either goserelin 36 or leuprolide acetate 37, for 18-24 weeks. The idea, said Dr. Eastham, is to target two different populations of cancer cells that might separately be sensitive to each of these different approaches. "If such a strategy were effective, it could significantly change clinical practice."
For More Information
See the list of eligibility criteria and contact information at http://www.cancer.gov/clinicaltrials/CALGB-90203 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The call is toll free and confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
Following are newly released NCI research funding opportunities:
Using Systems Science Methodologies to Protect and Improve Population Health Announcement Number: PA-08-224
Letter of Intent Receipt Date: Sept. 16, 2008
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3912. Inquiries: Dr. Stephen Marcus - sm311j@nih.gov.
Etiology, Prevention, and Treatment of Hepatocellular Carcinoma
Announcement Number: PAR-08-245
Letter of Intent Receipt Dates: Sept. 22 2008; Dec. 28, 2008; April 28, 2009; Aug. 29, 2009; Dec. 28, 2009; April 28, 2010; Aug. 28, 2010; Dec. 28, 2010; and April 30, 2011
Application Receipt Dates: Oct. 20, 2008; Jan. 28, 2009; May 28, 2009; Sept. 29, 2009; Jan. 28, 2010; May 28, 2010; Sept. 28, 2010; Jan. 28, 2011; and May 31, 2011
This is a renewal of PA-05-138 and will use the P01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3936. Inquiries: Dr. Elizabeth Read-Connole - bconnole@mail.nih.gov.
Exceptional, Unconventional Research Enabling Knowledge Acceleration
Announcement Number: RFA-GM-09-008
Letter of Intent Receipt Date: Sept. 29, 2008
Application Receipt Date: Oct. 28, 2008
This is a renewal of RFA-GM-08-002 and will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3933. Inquiries: Dr. Judy Mietz - mietzj@mail.nih.gov.
Fogarty International Research Collaboration - Behavioral and Social Sciences Research Award
Announcement Number: PAR-08-223
Application Receipt Dates: Sept. 29, 2008, Sept. 29, 2009, and Sept. 29, 2010
This is a renewal of PAR-06-437 and will use the R03 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3914. Inquiries: Dr. Michele Bloch - blochm@mail.nih.gov.
Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants
Announcement Number: PA-08-226
Application Receipt Dates: Non-AIDS Applications (new): Sept. 25, 2008; Jan. 25, May 25, and Sept. 25, 2009; Jan. 25, May 25, and Sept. 25, 2010; Jan. 25, and May 25, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This is a renewal of PA-06-468 and will use the T32 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3910. Inquiries: Dr. Lester S. Gorelic - gorelicl@mail.nih.gov.
Quantitative Imaging for Evaluation of Responses to Cancer Therapies
Announcement Number: PAR-08-225
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
This funding opportunity will use the U01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3911. Inquiries: Dr. Robert J. Nordstrom - nordstrr@mail.nih.gov.
Translational Research at the Aging/Cancer Interface
Announcement Number: PA-08-230
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3928. Inquiries: Dr. Susan G. Nayfield - nayfiels@mail.nih.gov.
Impact of Health Communication Strategies on Dietary Behaviors
Announcement Number: PA-08-239
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008, Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3932. Inquiries: Dr. Amy Yaroch - yarocha@mail.nih.gov.
Reducing Risk Behaviors by Promoting Positive Youth Development
Announcement Number: PA-08-241
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3934. Inquiries: Dr. Frank Perna - pernafm@mail.nih.gov.
Etiology, Prevention, and Treatment of Hepatocellular Carcinoma
Announcement Number: PA-08-243
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This is a renewal of PA-07-258 and will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3936. Inquiries: Dr. Elizabeth Read-Connole - bconnole@mail.nih.gov.
Metals in Medicine
Announcement Number: PA-08-251
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008, Feb. 5, June 5, and Oct. 5, 2009, Feb. 5, June 5, and Oct. 5, 2010, Feb. 5, and June 5, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This is a renewal of PA-07-276 and will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3937. Inquiries: Dr. Cindy D. Davis - Davisci@mail.nih.gov.
Integration of Mouse Models into Human Cancer Research
Announcement Number: RFA-CA-08-018
Letter of Intent Receipt Date: Oct. 14, 2008
Application Receipt Date: Nov. 14, 2008
This is a renewal of RFA-CA-04-002 and will use the U01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3938. Inquiries: Cheryl L. Marks - marksc@mail.nih.gov.
Translational Research at the Aging/Cancer Interface
Announcement Number: PA-08-231
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3928. Inquiries: Dr. Susan G. Nayfield - nayfiels@mail.nih.gov.
Impact of Health Communication Strategies on Dietary Behaviors
Announcement Number: PA-08-240
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008, Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3932. Inquiries: Dr. Amy Yaroch - yarocha@mail.nih.gov.
Reducing Risk Behaviors by Promoting Positive Youth Development
Announcement Number: PA-08-242
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This funding opportunity will use the R03 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3934. Inquiries: Dr. Frank Perna - pernafm@mail.nih.gov.
Etiology, Prevention, and Treatment of Hepatocellular Carcinoma
Announcement Number: PA-08-244
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, Sept. 7, 2011
This is a renewal of PA-06-295 and will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3936. Inquiries: Dr. Elizabeth Read-Connole - bconnole@mail.nih.gov.
Small Grants Program for Cancer Epidemiology
Announcement Number: PAR-08-237
Application Receipt Dates: March 19, 2009; July 17, 2009; November 19, 2009; March 19, 2010; July 23, 2010; November 19, 2010; March 18, 2011; July 22, 2011; November 18, 2011
This is a renewal of PAR-06-294 and will use the R03 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3931. Inquiries: Dr. Mukesh Verma - vermam@mail.nih.gov.
|
 |
 |
A Conversation with Dr. Diane E. Meier
 Dr. Diane E. Meier is director of the Center to Advance Palliative Care 38 (CAPC), a national organization devoted to increasing the number and quality of palliative care programs in the United States. She is also director of the Lillian and Benjamin Hertzberg Palliative Care Institute; professor of Medicine and Geriatrics; and Catherine Gaisman Professor of Medical Ethics at Mount Sinai School of Medicine in New York City.
Dr. Diane E. Meier is director of the Center to Advance Palliative Care 38 (CAPC), a national organization devoted to increasing the number and quality of palliative care programs in the United States. She is also director of the Lillian and Benjamin Hertzberg Palliative Care Institute; professor of Medicine and Geriatrics; and Catherine Gaisman Professor of Medical Ethics at Mount Sinai School of Medicine in New York City.
What are the challenges for palliative care in cancer?
One challenge is the widespread myth that palliative care is the same as end-of-life care. Palliative care is distinct from hospice care in that it is appropriate for any patient with a serious illness, regardless of their prognosis. For example, our hospital palliative care program delivers care to young people with curable leukemias and curable Hodgkin disease, who are going through a very difficult time at diagnosis and during the aggressive treatments that they need to cure their diseases. What we try to do is make the experience of a serious illness more bearable for the patients and their families.
A second major barrier is the intense focus on disease-specific treatment in American medicine and the highly specialized, fragmented care that results. Most cancer patients in this country are over age 65. Most of them have multiple chronic diseases with cancer being only one of them. When a person of any age has cancer their oncologist usually becomes their de facto primary physician. Yet the problems of cognitive and functional impairment, multi-morbidity, fatigue, frailty, risks of falling, and exhausted and overburdened family caregivers are not things that oncologists are set up to handle. With the partnership of a palliative care team, these kinds of problems can be addressed at the same time that cancer treatments are administered by the oncologist.
What is on CAPC's research agenda?
Our research agenda is quite broad. We are tracking the growth of palliative care programs to assess access around the country. We will publish a paper at the end of this month in the Journal of Palliative Medicine that shows enormous variability in access to hospital palliative care programs, just as in every other aspect of the health care system.
We're also doing NCI-funded research to document the impact of palliative care on quality of life, patient and family satisfaction, need for hospital-level care (such as number of emergency room visits and days spent in an intensive care unit), and costs and efficiencies of care. Another paper published this week in the Archives of Internal Medicine 5 demonstrates dramatic cost avoidance associated with palliative care in eight hospitals in low-, medium-, and high-cost markets.
Are there signs of progress in palliative care?
Palliative care is getting a lot of national recognition. The Joint Commission [an independent, not-for-profit organization that accredits and certifies health care organizations and programs in the United States] will soon launch a palliative care certificate program, in which hospitals apply to receive a certificate of quality for their palliative care programs. In addition, the National Quality Forum has published a framework and preferred practices for palliative care, so there are standards and guidelines to ensure the delivery of the highest possible quality of palliative care. Last year, the American Board of Medical Specialties approved palliative care as a sub-specialty with an unprecedented 11 "parent" specialty boards, including internal medicine, family medicine, and surgery. That's a real sign of the growing recognition of the value of palliative care for all kinds of patients with all kinds of medical problems.
|
 |
 |
President's Cancer Panel Focuses on Environment and Cancer
Beginning this month, the President's Cancer Panel 39 will hold a series of four meetings titled Environmental Factors in Cancer. These meetings will focus on cancer-causing pollutants found in air, soil, food, and water; exposure to toxic chemicals in the workplace; and radiation exposure, among other topics. The Panel will explore both current research and existing knowledge gaps in these areas, as well as the status of regulatory practices relevant to environmental factors and cancer risk reduction. The Panel will hear testimony from basic scientists, public health professionals, government representatives, and advocates.
The series begins September 16 in East Brunswick, NJ, where the focus will be on "Industrial and Manufacturing Exposures." Meetings are free and open to the public. To learn more about the series, go to http://pcp.cancer.gov.
NCI Director to Kick Off Teleconference Series
NCI's Office of Advocacy Relations 40 (OAR) begins its fall 2008 "Understanding NCI" teleconference series on September 17 from 1:00-2:00 p.m. ET, with an update for the advocacy community from NCI Director Dr. John E. Niederhuber. Mr. Doug Ulman, chair of the NCI Director's Consumer Liaison Group 41, will also be a featured speaker.
The teleconference can be accessed toll-free within the U.S. at 800-857-6584; the passcode is NCI. Toll-free playback will be available through October 17 at 800-934-9468. For more information and the teleconference schedule, go to http://advocacy.cancer.gov/activities/teleconferences or contact OAR at 301-594-3194.
Abstracts Accepted for Chromosome Biology Symposium
On October 30 and 31, NCI's Center for Cancer Research and the Center of Excellence in Chromosome Biology will host the "Genome-wide Chromatin Structure and Function" Symposium in the Natcher Conference Center on the NIH campus. Leading researchers from NCI and around the world will highlight recent advances, define novel directions of basic chromosome research, and discuss the use and implications of these advances for clinical use.
The deadline for abstract submission for the symposium poster session is September 24. Online registration for meeting participants ends October 15. For more information and to register for the symposium, please go to: http://www.blsmeetings.net/chromosomebio2008.

International Meeting Addresses Global Cancer Burden
The International Union Against Cancer (UICC) hosted the World Cancer Congress in Geneva, Switzerland, on August 27-31.
This year, 2,500 experts and
advocates from around the world, including NCI staff, focused on
transforming the latest knowledge into strategies for reducing the
cancer burden worldwide.
The scientific agenda covered global cancer prevention and control, tobacco control, knowledge transfer, supportive care, and capacity building. Other highlights included results from a UICC/Gallup poll on cancer risk factors, the cancer-themed Reel Lives film festival, and a full-day session with the International Cancer Information Service Group (ICISG).
Fifty participants from 18 countries attended a course presented by the ICISG on how to start and manage a cancer information service. NCI's Cancer Information Service and Office of Communications and Education also showcased products and services developed for international use.
NCI has a burgeoning international portfolio 42 and supports international activities through the NCI Office of International Affairs 43 and the NCI Liaison Office 44 in Brussels, Belgium.
Clinical Trials Participants Honored
On September 5, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation commemorated its 50th anniversary by donating a sculpture to honor the thousands of people who have participated in NSABP research and clinical trials. The sculpture, titled "Emergence," signifies the development of new and proven therapies, which are only possible with the contributions of clinical trial volunteers. The limestone sculpture will be on permanent display at the NIH Clinical Center in Bethesda, MD - the country's largest hospital devoted entirely to clinical research.
NCAB Meeting Held
The National Cancer Advisory Board met on September 7 and 8. To view a videocast of the public portions of the meeting, go to http://videocast.nih.gov/PastEvents.asp.
| |
 |
 |

NCI's Palliative Care Working Group
"Patients should not have to choose between treatment with curative intent or comfort care," the Institute of Medicine stated in its 2001 report 45 on palliative care in cancer treatment. "There is a need for both, in varying degrees, throughout the course of cancer, whether the eventual outcome is long-term survival or death." Following this with a series of 10 recommendations, the report called on NCI to play a prominent role in changing the practices and institutions that influence palliative care in the United States.
NCI's Palliative Care Working Group has risen to meet this challenge, with 17 members from offices across the institute working with allied agencies to understand how palliative care can be incorporated into routine cancer care, as well as identify areas where greater effort may be needed.
The group's progress and links to
participating offices are outlined under the "Research and Funding" section for symptom management 46 on Cancer.gov. The topics on this Web page include complementary and alternative medical practices, fertility preservation, mental health, and religious and spiritual support - notably, not limited to the context of end-of-life care, as palliative care is so frequently misunderstood to be.
Working group member Dr. Bryce Reeve reports that NCI is working with the Food and Drug Administration to develop a Patient-Reported Outcomes version of NCI's Common Terminology Criteria for Adverse Events (CTCAE), a system used to document adverse events during treatment. The Patient-Reported Outcomes version will capture patient-reported symptoms such as fatigue, pain, and nausea electronically and integrate these with the revised CTCAE to enhance safety monitoring in oncology trials.
Dr. Julia Rowland, who leads NCI's Office of Cancer Survivorship 47, notes that in 2008, her office promoted palliative care research and discussion at its biennial conference. Many of the talks that were given during the previous biennial conference 48 were presented this year in a supplemental issue of the journal Cancer 49. Her office also hosted a free teleconference series 50, and more than 1,700 cancer survivors from nine countries called in to hear expert-led discussions of palliative care and other survivorship topics.
Robin Baldwin, editorial board
content manager of NCI's Physician Data Query (PDQ) project, notes
that the PDQ Supportive and Palliative Care Editorial Board has produced 25 summaries 51 on palliative care issues. "Recently the board has added a new summary 52 on pediatric supportive care," she says. "This summary looks at psychological adjustment to cancer treatment in the pediatric patient, and it will be expanded to include information on physical adjustment, cognitive development, and long-term survivorship."
Another benchmark for the working group in 2008 is the successful dissemination of "Education in Palliative and End-of-Life Care for Oncology 53" (EPEC-O) by NCI's Office of Communications and Education (OCE). This multimedia, train-the-trainer curriculum was developed by Northwestern University, with funding from NCI and the Lance Armstrong Foundation.
EPEC-O was recently adapted for Native American populations, explains OCE's Dr. Cheryl Arenella, "and 89 health providers working within the Indian Health Service (IHS) have been trained to date, returning to their base practice site to train their peers and initiate new palliative services." Additional partnerships with IHS for palliative care education are being explored, she notes.
Updated publications for patients and caregivers are also available. English and Spanish versions of NCI's Pain Control - Support for People with Cancer booklet now include information and common misunderstandings about pain control, medicine, and side effects; communicating with health providers about pain; and psychosocial issues that arise with pain. These booklets and other materials are available on Cancer.gov. Free copies can be ordered online or via the NCI Cancer Information Service's toll-free number, 1-800-4-CANCER.
The NCI Palliative Care Working Group is coordinated by Andrea Denicoff of NCI's Division of
Cancer Treatment and Diagnosis 54.
For more information about the working group, contact her at
denicofa@mail.nih.gov.
—Brittany Moya del Pino
|
 |
|
Table of Links
| 1 | http://cancergenome.nih.gov |
| 2 | http://www.ncbi.nlm.nih.gov/pubmed/18772890 |
| 3 | http://www.cancer.gov/cancertopics/druginfo/temozolomide |
| 4 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_090908/page2 |
| 5 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_090908/page3 |
| 6 | http://www.cancer.gov/newscenter/pressreleases/TCGAglioblastoma |
| 7 | http://www.ncbi.nlm.nih.gov/pubmed/18772396 |
| 8 | http://www.ncbi.nlm.nih.gov/pubmed/18772397?ordinalpos=1&itool=EntrezSystem2.PE
ntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum |
| 9 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_091206/page2 |
| 10 | http://www.ncbi.nlm.nih.gov/pubmed/18695134 |
| 11 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_080508/page6 |
| 12 | http://www.ncbi.nlm.nih.gov/pubmed/18688056 |
| 13 | http://www.ncbi.nlm.nih.gov/pubmed/18716299 |
| 14 | http://www.cancer.gov/clinicaltrials/G-0034 |
| 15 | http://www.cancer.gov/cancertopics/druginfo/docetaxel |
| 16 | http://www.cancer.gov/researchandfunding/announcements/symptommanagement#Closed
+Requests+for+Applications+(RFA)+from+NCI |
| 17 | http://prevention.cancer.gov/programs-resources/groups/copt/programs/active#RFA |
| 18 | http://ccct.nci.nih.gov/ccct/steering-committees/smsc |
| 19 | http://dcp.cancer.gov/programs-resources/programs/ccop |
| 20 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=46384 |
| 21 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=467867 |
| 22 | http://www.ncbi.nlm.nih.gov/pubmed/18724359 |
| 23 | http://ctep.cancer.gov |
| 24 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_112106/page3 |
| 25 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_051308/page2 |
| 26 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=46283 |
| 27 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45762 |
| 28 | http://www.cancer.gov/dictionary/?searchTxt=radical+prostatectomy&btnGo.x=0&btn
Go.y=0&sgroup=Starts+with&lang= |
| 29 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=46540 |
| 30 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45696 |
| 31 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45800 |
| 32 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=350224 |
| 33 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=301626 |
| 34 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=46138 |
| 35 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45382 |
| 36 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45289 |
| 37 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=595341 |
| 38 | http://www.capc.org |
| 39 | http://pcp.cancer.gov |
| 40 | http://advocacy.cancer.gov |
| 41 | http://dclg.cancer.gov |
| 42 | http://www.cancer.gov/nci-international-portfolio |
| 43 | http://oia.cancer.gov |
| 44 | http://ncilobrussels.cancer.gov |
| 45 | http://books.nap.edu/catalog.php?record_id=10147 |
| 46 | http://www.cancer.gov/researchandfunding/announcements/symptommanagement |
| 47 | http://dccps.nci.nih.gov/ocs |
| 48 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_062408/page4 |
| 49 | http://www.ncbi.nlm.nih.gov/pubmed/18428200 |
| 50 | http://cancercontrol.cancer.gov/ocs/telephone_edu_mtgs.html |
| 51 | http://www.cancer.gov/cancertopics/pdq/supportivecare |
| 52 | http://www.cancer.gov/cancertopics/pdq/supportivecare/pediatric/healthprofessio
nal |
| 53 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_112806/page10 |
| 54 | http://dctd.cancer.gov |
|
 |