|
1: The Clinical Trial Process
What Are Clinical Trials
Cancer Clinical Trials - Types and Phases
Cancer Treatment Trials
Cancer Prevention Trials
Other Types of Cancer Clinical Trials
How Participants Are Assigned in Randomized Trials
The Clinical Trial Protocol
Exercises
Clinical trials are research studies involving
people.They seek to answer specific scientific questions to
find better ways to prevent, detect, and treat diseases, and
to improve care for people with diseases. Clinical trials
differ by type of trial and phase of trial. Each clinical
trial follows a set of strict scientific guidelines called a
protocol.
|
Learning Objectives
By reading this section and completing the exercises, you
will be able to:
Define clinical trials
Name the different types and phases of clinical
trials
Describe how participants are assigned to groups in
"randomized" clinical trials
Review the purpose of a clinical trial protocol and
its importance
Dispel common myths about clinical trials
|
Clinical trials are research studies involving people. They are
the final step in a long process that begins with preliminary
laboratory research and animal testing. Clinical trials try to answer
specific scientific questions to find better ways to prevent, detect,
or treat diseases or to improve care for people with diseases.
In cancer research, a clinical trial is designed to show how a
certain anticancer approach - for instance, a promising drug, a new
surgical procedure, a new diagnostic test, or a possible way to
prevent cancer - affects the people who receive it.
Types of Clinical Trials
There are several different types of cancer clinical trials. This
workbook will focus primarily on cancer treatment and prevention
trials. Each type of trial is designed to answer different research
questions:
Phases of Clinical Trials
Trials take place in four phases, each designed to answer
different research questions.
|
filler
|
Phase
1
|
Phase
2
|
Phase
3
|
Phase
4
|
|
Number of participants
|
15-30 people
|
Less than 100 people
|
Generally, from 100 to thousands of people
|
Several hundred to several thousand people
|
|
Purpose
|
To find a safe dosage
To decide how the agent should be given
To observe how the agent affects the human
body
|
To determine if the agent or intervention has an
effect on a particular cancer
To see how the agent or intervention affects the
human body
|
To compare the new agent or intervention (or new
use of a treatment) with the current standard
|
To further evaluate the long-term safety and
effectiveness of a new treatment
|
The trial phases are explained in the context of drug treatment
trials, but the same concepts apply to most types of clinical
trials.
Most cancer clinical trials are treatment studies. These clinical
trials involve people who have cancer. Treatment studies are designed
to answer specific questions about and evaluate the effectiveness of
a new treatment or a new way of using an old treatment. These trials
test many types of treatments, such as new drugs, vaccines, new
approaches to surgery or radiation therapy, or new combinations of
treatments.
Phase 1: Looking at Safety
Once laboratory studies show that a new approach has promise, a
phase 1 trial can begin. A phase 1 trial is the first step in testing
a new cancer agent in humans.
In these studies, researchers look for the best way to give people
the new agent (for example, by pill or by injection), how often it
should be given, and what the safest dose is. These studies also
include special laboratory tests such as blood tests and biopsies to
evaluate how the new agent is working in
the body.
|
An agent is a substance
that produces, or that researchers believe
is capable of producing, an effect that fights
cancer.
|
In phase 1 cancer trials, small groups of people with cancer
are treated with a certain dose of a new agent that has already
been extensively studied in the laboratory. During the trial, the
dose is usually increased group by group in order to find the
highest dose that does not cause unacceptable harmful side
effects, called toxicity. This process determines a safe and
appropriate dose to use in a phase 2 trial. Although the primary
purpose of phase 1 trials is to find the safest dose of a new
agent, researchers also evaluate whether the new agent benefits
people.
|
Toxicity refers to harmful side effects caused
by the agent or intervention being tested.
|
Who Participates in Phase 1 Treatment Trials?
People with cancer who are eligible for phase 1 studies have no
known effective treatment options, or they have already tried other
treatment options. Many participate in these trials because they want
to help others and contribute to cancer research. Phase 1 cancer
trials usually have 15 to 30 participants.
What Are the Benefits and Risks for Phase 1 Treatment Trial
Participants?
Benefits
If the new agent under study has an effect on the cancer,
participants may be among the first to benefit.
Risks
Because most phase 1 trials are testing agents for the
first time in humans, unpredictable side effects can occur.
Phase 2: How Well the New Treatment Works
Phase 2 trials continue to test the safety of the new agent, and
begin to evaluate how well it works against a specific type of
cancer. In these trials, the new agent is given to groups of people
with one type of cancer or related cancers, using the dosage found to
be safe in phase 1 trials.
Who Participates in Phase 2 Trials?
In general, people with cancer who take part in phase 2 trials
have been treated with chemotherapy, surgery, or radiation, but the
treatment has not been effective. Participation in these trials is
often restricted based on the previous treatment received. Phase 2
cancer trials usually have less than 100 participants.
What Are the Benefits and Risks for Phase 2 Treatment Trial
Participants?
Benefits
If the new agent under study has an effect on the cancer,
participants may be among the first to benefit.
Risks
It is important to remember that when a phase 2
trial begins, it is not yet known if the agent tested works
against the specific cancer being studied.
Unpredictable side effects can also occur in these
trials.
Phase 3: Comparing a New Treatment to the Standard Treatment
Phase 3 trials focus on learning how a new treatment compares to
standard, or the most widely accepted, treatment. Researchers want to
learn whether the new treatment is better than, the same as, or worse
than the standard treatment.
In phase 3 trials, participants have an equal chance to be
assigned to one of two or more groups (also called "arms"). In a
study with two groups:
Placebos are almost never used in cancer treatment trials. In rare
cases in which no standard treatment exists for a cancer, some
studies compare a new treatment with a placebo.
|
A placebo is designed to look like the medicine
being tested but doesn't contain any active ingredient.
Some people call a placebo a "sugar pill."
|
The process of assigning participants to groups is called
randomization. Additional discussion of randomization is included
later in this section.
|
Finding Out About Standard Cancer Care
The National Cancer Institute's Web site www.cancer.gov
contains the latest information about standard cancer
treatment, screening, prevention, genetics, supportive care,
and complementary and alternative medicine, as well as a
registry of cancer clinical trials. Most cancer information
summaries appear in two versions: a technical version for
the health professional and a nontechnical version for the
public. Many of the summaries are also available in
Spanish.
|
Who Participates in Phase 3 Trials?
Participants in phase 3 studies range from people newly diagnosed
with cancer to people with extensive disease. Phase 3 studies are
designed to answer research questions across the disease continuum.
Phase 3 trials usually have hundreds to thousands of participants, in
order to find out if there are true differences in the effectiveness
of the treatment being tested.
What Are the Benefits and Risks for Phase 3 Treatment Trial
Participants?
Benefits
Regardless of the group a participant is assigned
to, he or she will receive, at a minimum, the best standard
treatment.
If a participant is taking the new treatment and it is
proven to work better than the standard treatment, he or she may
be among the first to benefit.
Risks
New treatments under study do not always turn out
to be better than, or even as good as, standard treatment.
New treatments under study may have side effects that
are worse than those of standard treatment.
Despite phase 1 and 2 testing, unexpected side effects
may occur.
Like standard treatments, new treatments may not work
for every participant.
A participant might receive standard treatment, which
might be found to be less effective than the new approach.
Phase 4: Continuing Evaluation
Phase 4 trials are used to further evaluate the long-term safety
and effectiveness of a treatment. Less common than phase 1, 2, and 3
trials, phase 4 trials take place after the new treatment has been
approved for standard use.
|
Biological Therapies: Finding Out How the Immune
System Works Against Cancer
Biological therapy (sometimes called immunotherapy,
biotherapy, or biological response modifier therapy) uses
the body's immune system, either directly or indirectly, to
fight cancer or to lessen the side effects that some cancer
treatments might cause.
The immune system is a complex network of cells and
organs that work together to defend the body against attacks
by "foreign" or "non-self" invaders. This network is one of
the body's main defenses against disease. It works against
disease, including cancer, in a variety of ways.
Biological therapies are designed to repair, stimulate,
or enhance the immune system's responses. Many clinical
trials are now testing the use of biological therapies, such
as monoclonal antibodies and vaccines, to fight cancer.
Monoclonal Antibodies (MOABs)
Monoclonal antibodies are a form of biological therapy
that is now being studied in the laboratory and in clinical
trials. MOABs may help the body's own immune system fight
cancer by locating cancer cells and either killing them or
delivering cancer-killing substances to them without harming
normal cells.
Cancer Vaccines
Cancer vaccines are another form of biological therapy
now being studied in the laboratory and in clinical
trials.
Researchers are developing vaccines that may help a
person's immune system recognize cancer cells. These
vaccines may help the body reject tumors and prevent cancer
from recurring. In contrast to vaccines against infectious
diseases, cancer vaccines are designed to be injected after
the disease is diagnosed, rather than before it develops.
Cancer vaccines given when the cancer is small may be able
to eradicate the cancer.
Many vaccines are not used alone, but in combination with
other treatments such as surgery, chemotherapy, or
additional interactions that help stimulate the immune
response in general.
|

Unlike treatment trials, cancer prevention trials are studies
involving healthy people who are at high risk for developing cancer.
These studies try to answer specific questions about cancer risk and
evaluate the effectiveness of ways to reduce cancer risk. They look
at approaches to preventing cancer from developing in people who have
not previously had cancer.
There are two kinds of prevention trials.
Action studies
("doing something") focus on finding out whether actions people
take - such as exercising more or quitting smoking - can prevent
cancer. Agent studies
("taking something") focus on finding out whether taking
certain medicines, vitamins, minerals, or food supplements (or a
combination of them) may lower the risk of a certain type of
cancer. Agent studies are also called chemoprevention
studies.
Researchers who conduct these studies want to know:
How Chemoprevention Trials Work
Chemoprevention trials also go through phases. In phase 3 agent
studies:
Placebos are used in prevention trials when there is not yet a
known approach or standard agent for cancer prevention in the
population being studied. It is important to remember that
participants in prevention trials do not have cancer.
Who Participates in Prevention Trials?
Prevention trials include people who may be at risk for developing
cancer. Many chemoprevention trials require that participants be at
high risk for developing cancer.
What Are the Benefits and Risks for Prevention Trial
Participants?
Benefits
Risks
New cancer prevention drugs or interventions may
have unknown side effects or risks.
The side effects of the drug or intervention may be
worse and the effectiveness less than those of standard preventive
measures.
Even if a new drug or intervention is effective, it may
not work for every participant.
Early Detection/Screening Trials
The goal of early detection/screening trials is to discover
methods for finding cancer as early as possible. For many types of
cancer, detecting and treating the disease at an early stage can
result in an improved outcome - a better chance to shrink the tumor,
minimize its effects, or cause it to go away completely.
Ways to find cancer include:
Diagnostic Trials
These trials focus on how new tests or procedures can better
identify whether people have cancer. Diagnostic tests or procedures
are done to find out whether cancer is present and, if so, where it
is located in the body, if it has spread, and how much cancer is
there.
Some diagnostic trials compare two or more techniques to diagnose
cancer, find out how accurate they are, and see whether they can
provide any new and valuable information about someone's cancer.
Genetic tests are being evaluated as diagnostic tools to further
classify cancers, which may help direct therapies or improve
treatments for people with specific genetic changes.
Quality-of-Life/Supportive Care Trials
These trials evaluate improvements in the comfort and quality of
life of people who have cancer. They find ways to help people who are
having nutrition problems, infection, nausea and vomiting, sleep
disorders, depression, or other effects from cancer and/or its
treatment. Some supportive care trials focus on families and
caregivers to help them cope with both their own needs and those of
the person with cancer.

|
Genetics Research
Genetics studies may be a part of any cancer clinical
trial and focus on understanding how someone's genetic
make-up can assist in the early detection, diagnosis, or
treatment of cancer. Genetic research is also being used to
help develop future cancer treatments.
Population and family-based genetic research studies
differ from the traditional cancer clinical trials. In these
studies, researchers look at tissue or blood samples, either
from families or large numbers of people, to find genetic
changes that are associated with cancer. These people may or
may not have cancer. The goal of these studies is to help
understand what causes cancer.
Genetics research is an important part of cancer research
because it contributes to the knowledge of the causes of
cancer and can lead to developing new clinical trials that
focus on cancer prevention, detection, and treatment.
|
Phase 3 studies are randomized clinical trials. Some phase 2
trials may also be randomized.
Randomization is a method used to prevent bias in research. In
phase 3 studies (and some phase 2 studies), participants are assigned
to either the investigational group or the control group by chance,
via a computer program, or with a table of random numbers.
Randomization ensures that unknown factors do not influence the trial
results.
The control group is made up of people who will
get the most widely accepted treatment (standard treatment) for
their cancer.
The investigational group is made up of people who will
get the new agent or intervention being tested.
Anyone who is considering participation in a randomized clinical
trial needs to understand that she or he has an equal chance to be
assigned to one of the groups. The doctor does not choose the group
for the participant.
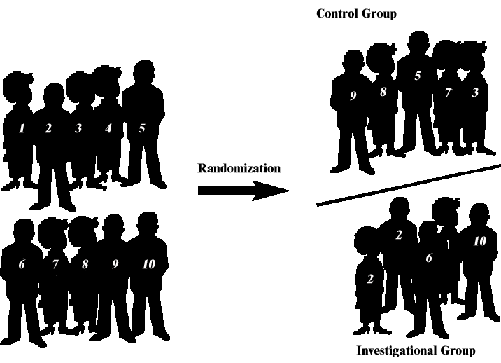
Randomization is a method used to prevent bias in research. A
computer or a table of random numbers generates treatment assignments
and participants have an equal chance to be assigned to one of two or
more groups (e.g., the control group or the investigational
group)
|
Bias can be human
choices, beliefs, or any other factors besides those
being studied which can affect a clinical trial's
results.
|
Why Is Randomization Important?
If participants or doctors choose a particular group based on what
they think is best, then one of the groups would likely be very
different than the other, making comparison between the groups
difficult. Randomization eliminates this bias because participants
have an equal chance of being assigned to either group and the
subgroups are as similar as possible. Comparing similar groups of
people taking different treatments for the same type of cancer is a
way to ensure that the study results are caused by the treatments
rather than by chance or other factors.
Clinical trials follow strict scientific guidelines. These
guidelines clearly state the study's design and who will be able to
participate in the study. Every trial has a person in charge, usually
a doctor, who is called the principal investigator. The principal
investigator prepares a plan for the study, called a protocol, which
acts like a "recipe" for conducting a clinical trial.
The protocol explains what the trial will do, how the study will
be carried out, and why each part of the study is necessary. It
includes information on:
The reason for doing the study
How many people will be in the study
Who is eligible to participate in the study
(requirements might involve type of cancer, general health,
age)
Any agents participants will take, the dosage, and how
often
What medical tests participants will have and how
often
What information will be gathered about the
participants
The endpoints of the study
|
Endpoints
An endpoint is what researchers will measure to evaluate
the results of a new treatment being tested in a clinical
trial. Research teams establish the endpoints of a trial
before it begins.
It is important to note that endpoints differ, depending
on the type and phase of the clinical trial. Examples of
endpoints are:
Toxicity
Tumor response
Survival
Quality of life
|
|
Every doctor or research center that takes part in the
trial uses the same protocol. This ensures that participants
are treated identically no matter where they are receiving
treatment so the information from all the participating
sites can be combined and compared.
Although some people may choose to read the entire
protocol before they choose to participate, the law requires
that, at a minimum, participants learn about the study
protocol through a process called informed consent. This
information helps individuals decide whether to
participate.
|
|
Guidelines on Who Can Join a Trial
Depending on the research questions, each clinical trial
protocol clearly states the type of person who can or cannot
participate in the trial. The reason for these guidelines
includes ensuring:
Other common qualifications for entering a trial
include:
Having a certain type or stage of cancer
Having been previously treated with a certain
kind of therapy
Being in a certain age group
These criteria help ensure that trial participants are as
similar as possible so doctors are confident that the
reasons for the results are due to the agent or intervention
being studied and not other factors.
|
|
|

|
These exercises are optional. They are provided as tools to review the information presented in each chapter.
A. Cancer treatment clinical trials are the treatment of last
resort.
_____________________________________________________
_____________________________________________________
_____________________________________________________
B. Only people who have cancer are eligible to participate in a
cancer clinical trial.
_____________________________________________________
_____________________________________________________
_____________________________________________________
C. Many people who join cancer treatment clinical trials get a
sugar pill (placebo) instead of being treated.
_____________________________________________________
_____________________________________________________
_____________________________________________________
D. By restricting who can go on trials, investigators keep people
from getting a new treatment that could save their lives.
_____________________________________________________
_____________________________________________________
_____________________________________________________
Answers to Exercise 1.1
Apply what you've learned about clinical trials to an actual
clinical trial protocol.
The following is an example of an abbreviated "patient version"
protocol taken from PDQ® (physician data query), a clinical trial
database sponsored by the National Cancer Institute. A "health
professional" version is also available for every trial.
Please note that this trial protocol is used for illustrative
purposes only; it may no longer be open to participants at the time
you are reading this guide.
Although some clinical trial participants may choose to read the
entire protocol before they choose to participate, the law requires
that participants learn about the study protocol through a process
called informed consent. This information helps them decide whether
to participate.
Directions
Read the following protocol and answer these questions. You may
want to print these questions for a reference.
A. What type of trial is this?
_____________________________________________________
_____________________________________________________
_____________________________________________________
B. What phase trial is this?
_____________________________________________________
_____________________________________________________
_____________________________________________________
C. Is it randomized?
_____________________________________________________
_____________________________________________________
_____________________________________________________
D. If someone meets the eligibility requirements, what kind of
person might be interested in participating?
_____________________________________________________
_____________________________________________________
_____________________________________________________
E. What might be of concern for someone considering participating
in this trial?
_____________________________________________________
_____________________________________________________
_____________________________________________________
Note that the protocol contains some blanks to indicate words
omitted for the purpose of this exercise.
Answers to Exercise 1.2
Protocol ID: NSABP-B-31
Phase _______, ________Study of Doxorubicin and Cyclophosphamide
Followed by Paclitaxel With or Without Trastuzumab (Herceptin) in
Women With Node Positive Breast Cancer That Overexpresses HER2
(Summary Last Modified 09/2000)
Patient Abstract
Rationale: Drugs used in chemotherapy use different ways to
stop tumor cells from dividing so they stop growing or die.
Monoclonal antibodies such as trastuzumab can locate tumor
cells and either kill them or deliver tumor-killing substances to
them without harming normal cells.
It is not yet known whether combination chemotherapy plus
trastuzumab is more effective than combination chemotherapy alone for
treating breast cancer.
Purpose: _________ phase _______ trial to compare the
effectiveness of combination chemotherapy with or without trastuzumab
in treating women who have stage I, stage II, or stage IIIA breast
cancer that has spread to lymph nodes in the armpit.
Eligibility:
• No more than 9 weeks since diagnosis
• Previous surgery to remove the tumor and lymph nodes in the
armpit
• No previous biological therapy, chemotherapy, hormone
therapy, or radiation therapy for breast cancer
Treatment: Patients will be randomly assigned to one of two
groups. Patients in group one will receive infusions of
doxorubicin and cyclophosphamide every 3 weeks for four
courses. About 3 weeks after the last course, these patients will
receive an infusion of paclitaxel every 3 weeks for four courses.
Patients in group two will receive chemotherapy as in group one.
They will also receive an infusion of trastuzumab on day 1 of the
first course of paclitaxel. They will continue to receive an infusion
of trastuzumab once a week for 51 weeks. Some patients will receive
tamoxifen by mouth once a day for 5 years. Some patients may receive
radiation therapy daily for 5-6 weeks. All patients will receive
follow-up evaluations every 6 months for 5 years and once a year
thereafter.
|
Key Protocol Terms
chemotherapy: Treatment with anticancer drugs.
monoclonal antibodies: Laboratory-produced
substances that can locate and bind to cancer cells wherever
they are in the body.
doxorubicin: An anticancer drug that belongs to
the family of drugs called antitumor antibiotics. It is an
anthracycline.
cyclophosphamide: An anticancer drug that belongs
to the family of drugs called alkylating agents.
infusion: A method of putting fluids, including
drugs, into the bloodstream. Also called intravenous
infusion.
tamoxifen: An anticancer drug that belongs to the
family of drugs called antiestrogens. Tamoxifen blocks the
effects of the hormone estrogen in the body. It is used to
prevent or delay the return of breast cancer or to control
its spread.
|
Back to Top
< Previous Section | Next Section > |