


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
 |
 |
||||
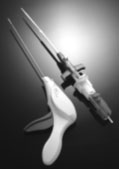 EVS™
Vascular Closure System - P040022
EVS™
Vascular Closure System - P040022This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: EVS™ Vascular Closure System
Applicant: angioLINK Corporation
Address: 125 John Hancock Road, Suite Six, Taunton, MA 02780
Approval Date: November 3, 2004
Approval Letter: http://www.fda.gov/cdrh/pdf4/p040022a.pdf
What is it? The EVS™ Vascular Closure System is used
to stop bleeding following diagnostic and interventional heart (cardiac) catheterization
procedures. After a cardiac catheterization procedure it is necessary to stop
the bleeding in a blood vessel in the leg (the femoral artery). The EVS™
System uses a titanium staple to stop bleeding by closing the hole in the artery.
During a cardiac catheterization procedure:
How does it work?
When is it used? The EVS™ System is used following a cardiac catheterization procedure. For a picture of a cardiac catheterization, use the following link: http://www.nlm.nih.gov/medlineplus/ency/imagepages/1080.htm
What will it accomplish? The EVS™ System is an alternative to applying manual pressure (compression) to the puncture site to stop bleeding. The EVS™ System uses a titanium staple to close the hole in the femoral artery and stop bleeding. The EVS™ System allows patients to get out of bed and walk sooner than is possible with standard compression methods.
When should it not be used? There are no contraindications for the use of this device.
Additional information: Summary of Safety and Effectiveness and labeling will be available at: http://www.fda.gov/cdrh/pdf4/p040022.html
Other:
Updated November 8, 2004
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH