


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
 |
 |
||||
 Syncardia
Temporary CardioWest Total Artificial Heart (TAH-t) - P030011
Syncardia
Temporary CardioWest Total Artificial Heart (TAH-t) - P030011This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: Syncardia temporary CardioWest™ Total
Artificial Heart (TAH-t)
Applicant: SynCardia Systems, Inc.
Address: 1992 E. Silverlake Road, Tucson, AZ 85713
Approval Date: October 15, 2004
Approval Letter: http://www.fda.gov/cdrh/PDF3/P030011a.pdf
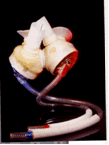
What is it? Syncardia temporary CardioWest™ Total Artificial Heart (TAH-t) is an implantable artificial heart intended to keep hospitalized patients alive while they are waiting for a heart transplant.
The temporary CardioWest™ TAH-t is a pulsating bi-ventricular device that is implanted into the chest to replace the patient's left and right ventricles (the bottom half of the heart). The device is sewn to the patient's remaining atria (the top half of the heart). Hospitalized patients are connected by tubes from the heart through their chest wall to a large power-generating console, which operates and monitors the device.
How does it work? The temporary CardioWest™ TAH-t is designed to duplicate the action of the natural heart ventricles.
When is it used? The temporary CardioWest™ TAH-t is used only in the hospital. The device is used as a "bridge to transplant" for patients waiting for a heart transplant who:
What will it accomplish? The temporary CardioWest™ TAH-t is used in patients to:
In a clinical study, 79 percent of patients implanted with the temporary CardioWest™ TAH-t received a donor heart with an average mean time of device implantation of 79 days, demonstrating that the artificial heart could successfully serve as a bridge to transplant.
When should it not be used? The temporary CardioWest™ TAH-t should not be used in patients who:
Additional information:
Summary of Safety and Effectiveness and labeling is available at: http://www.fda.gov/cdrh/PDF3/P030011b.pdf
Other:
FDA Talk Paper:
http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01317.html
HeartHealth Online:
www.fda.gov/hearthealth
Manufacturer’s product website:
http://www.syncardia.com
November 3, 2004
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH