


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
 |
 |
||||
FDA approved this device under the Humanitarian Device Exemption (HDE) program. See the links below to the Summary of Safety and Probable Benefit (SSPB) and other sites for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Wingspan™ Stent System with Gateway™
PTA Balloon Catheter
Manufacturer: Boston Scientific SMART
Address: 2401 Merced Street, San Leandro, California 94577
Approval Date: August 3, 2005
Approval Letter: http://www.fda.gov/cdrh/pdf5/h050001a.pdf
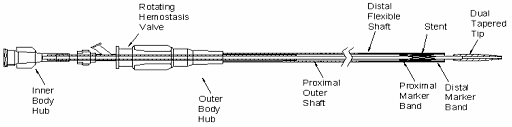
What is it? The Wingspan™ Stent System with Gateway™ PTA Balloon Catheter is used to open blocked arteries in the brain, allowing blood to flow to the brain. It consists of a Stent, a Delivery System, and a Balloon Catheter. The Balloon Catheter is used to open the blockage in brain arteries prior to inserting the stent. The Stent is the permanent implant that is placed in the artery to open the blockage. The Stent is a self-expanding, metal (nitinol) mesh in the shape of a tube. The Delivery System is a small catheter and is provided with the Stent pre-loaded. The Balloon Catheter is a small catheter with a tiny balloon on the tip.
How does it work?
When is it used? The Wingspan™ Stent System with Gateway™ PTA Balloon Catheter is used to open blocked arteries in the brain of patients after clot-dissolving drugs have been shown to not work.
What will it accomplish? The Balloon Catheter opens the blocked artery to improve blood flow. The Stent provides a barrier to reduce the risk of recurrent blockage or narrowing of the artery and to support the artery wall.
When should it not be used? The Wingspan™ Stent System with Gateway™ PTA Balloon Catheter should not be used in patients who:
Additional information:
SSPB and Labeling: http://www.fda.gov/cdrh/pdf5/h050001b.pdf
Updated September 2, 2005
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH