


   |
| FDA Home Page | CDRH Home Page | Search | A-Z Index |  |
|
 |
 |
||||
 REALIZE™ Band - P070009
REALIZE™ Band - P070009
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: REALIZE ™ Adjustable Gastric Band (REALIZE Band)
PMA Applicant: Ethicon Endo-Surgery, Inc.
Address: 4545 Creek Road , Cincinnati, Ohio 45242-2839
Approval Date: September 28, 2007
Approval Letter: http://www.fda.gov/cdrh/pdf7/p070009a.pdf
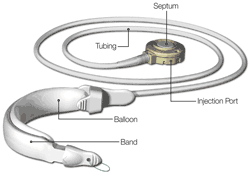
What is it? The REALIZE™ Band is a surgically implanted device used to help a person lose weight. The REALIZE™ Band consists of a silicone band, tubing, and an injection port.
How does it work? During the surgical procedure, the REALIZE™ Band is placed around the upper part of the stomach, creating a small stomach pouch that can hold only a small amount of food. The narrowed opening between the stomach pouch and the rest of the stomach controls how quickly food passes from the pouch to the lower part of the stomach. The band helps the person eat less by limiting the amount of food that can be eaten at one time and increasing the time it takes for food to be digested. The size of the band can be adjusted during a doctor’s office visit by adding or removing fluid from within the band via the injection port and tubing. These adjustments change the size of the narrowed opening. Inflating the band makes the opening smaller, causing food to pass more slowly. Deflating the band makes the opening larger, permitting food to pass more quickly. The injection port is placed under the skin on the chest wall. The port is connected to the band by the tubing.
When is it used? The REALIZE™ Band is used for weight loss in severely obese adults who have been obese for at least five years and for whom non-surgical weight loss methods have not been successful. They must be willing to make major changes in their eating habits and lifestyle. To be eligible for the band, a person must have a Body Mass Index (BMI) of at least 40, or a BMI of at least 35 with one or more severe comorbid conditions.
What will it accomplish? The band may help a person lose weight. In the U.S. study, the average weight loss was 42% of a person’s excess weight three years after the device was surgically implanted. Almost three quarters of the study subjects lost at least 25% of their excess weight; some lost over 75%, but others lost no weight. Weight loss may improve health problems associated with obesity such as high blood pressure or type 2 diabetes. At least one side effect (e.g., nausea and vomiting, port site pain, or gastroesophageal reflux disease) occurred in most subjects. The most serious side effects required either another operation (e.g., to replace or remove the band) or hospitalization to treat problems such as infection or dehydration due to severe vomiting.
When should it not be used? It should not be used for a person who is a poor candidate for surgery, has certain stomach or intestinal disorders, has an infection, or is being treated with steroids. It should not be used if a person is unable or unwilling to follow dietary restrictions.
Additional information: The Summary of Safety and Effectiveness and labeling will be available at: http://www.fda.gov/cdrh/pdf7/p070009.html
Updated October 24, 2007
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH