


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
 |
 |
||||
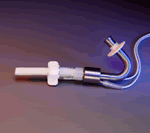
DeBakey VAD® Child -H030003 |
 |
FDA approved this device under the Humanitarian Device Exemption (HDE) program . See the links below to the Summary of Safety and Probable Benefit (SSPB) and other sites for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: DeBakey VAD® Child
Manufacturer: MicroMed Technology, Inc.
Address: 8965 Interchange Dr., Houston, TX 77054 USA
Approval Date: February 25, 2004
Approval Letter: http://www.fda.gov/cdrh/pdf3/H030003a.pdf
What is it? The DeBakey VAD® Child is a miniaturized heart pump designed to help the left ventricle of the heart pump blood. It is intended for use in children 5 to 16 years old who are awaiting a heart transplant.
How does it work? The DeBakey VAD® Child uses electromagnetic energy to cause a single component to spin around its axis. The spinning motion of this component works to propel blood from the left ventricle of the heart into the ascending aorta and circulate it throughout the body.
When is it used? The DeBakey VAD® Child is a Humanitarian Use Device (HUD) intended for both home and hospital use in children who:
What will it accomplish? Use of the DeBakey VAD® Child may allow children with severe left ventricular failure to survive long enough to receive a heart transplant.
When should it not be used? The DeBakey VAD® Child should not be used in children who:
Additional information: SSPB and Labeling: http://www.fda.gov/cdrh/ode/H030003sum.html
Other:
An FDA Talk Paper on the DeBakey VAD® Child:
http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01280.html
American Heart Association information on left ventricular assist devices:
http://www.americanheart.org/presenter.jhtml?identifier=4599
Ventricular assist device information from the Cedars-Sinai Heart Center:
http://www.csmc.edu/2355.html
National Heart, Lung, and Blood Institute facts about heart transplants:
http://www.nhlbi.nih.gov/health/public/heart/other/hrt_lung.htm
Updated March 10, 2004
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH