Home > Health & Education > eAdvances
New Adhesives for Damaged Joints: February 29, 2008
Millions of people suffer from joint pain caused by damaged cartilage, the lubricating tissue found on the ends of bones in various places in the body, including joints. The main sources of cartilage damage are sports injuries and arthritis. In arthritis, joint cartilage permanently wastes away, making even simple movements very painful. For many patients, relief comes only from total joint replacement, a surgical procedure that carries risks, including blood clots and nerve injury. An alternative to total joint replacement is cartilage repair. Being slippery, cartilage is particularly difficult to repair. Currently available adhesives for such repair have limitations. Synthetic adhesives are not highly compatible with tissues, and their biological counterparts – which are compatible with tissues – do not have sufficient binding strength to work on cartilage. Jennifer Elisseeff, Associate Professor of Biomedical Engineering, and her research team at Johns Hopkins University are exploring new ways of bonding cartilage tissue. They have modified a natural sugar substance called chondroitin sulfate (CS) to enable it to “glue” a jellylike material called hydrogel to cartilage tissue. Hydrogels, such as the material soft contact lenses are made of, are ideally suited for tissue engineering because they integrate well with the body, support nutrient-waste exchange, and promote new tissue growth. These synthetic materials can also be infused with nutrients and cells. Elisseeff’s CS adhesive, which chemically connects hydrogel to cartilage, is simple to administer and is not harmful to the cartilage tissue. CS is an attractive choice for tissue repair due to its anti-inflammatory activity, improvement of wound healing, and ability to absorb nutrients and water – which accounts for up to 80% of cartilage mass. CS reduces inflammation by several mechanisms, including diminishing the activity of white blood cells in the joint, and promotes healing by preventing cartilage degradation and stimulating production of proteoglycans, one of the building blocks of cartilage.
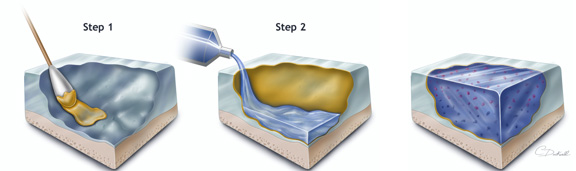
Engineering Cartilage GrowthElisseeff first tested the new adhesive in the laboratory, using a piece of cartilage taken out of the body. She applied a layer of CS to the damaged cartilage tissue and added a hydrogel layer containing cartilage cells (chondrocytes). Using sophisticated imaging technologies, Elisseeff verified that the CS adhesive was attached to and integrated with the cartilage. The cells in the hydrogel grew and secreted cartilage components, forming new tissue that bound the hydrogel with the old cartilage. In order to transfer her novel technology from the test tube to the clinic, Elisseeff combined the CS adhesive with a time-tested surgical procedure for joint repair – microfracture. Rather than transplanting cells, which is very costly and carries a risk of infection, microfracture enables using one’s own stem cells to regenerate defective tissue. In this procedure, small holes are drilled in the tissue underlying the damaged area of the cartilage. Through these holes, bone marrow stem cells carried by blood migrate to the bone surface where they eventually start producing new cartilage tissue. When tested in goat knees, the CS-hydrogel implants significantly improved the extent of cartilage repair over a period of 6 months, compared with untreated microfractures. New Adhesive to Enter the ClinicA similar approach is currently being tested in a Phase I clinical trial in people with knee injuries. “All of the patients are doing well, but it is too soon to tell whether the new adhesive improves the rate of cartilage repair. One thing that is nice with our material is that it discourages formation of scar tissue, which often forms after microfracture alone. So you have people that get initial relief of symptoms because they have tissue forming in that defect, but that is what is called scar-like cartilage, instead of real cartilage,” explains Elisseeff. Her collaborator, Dr. Norman Marcus, an orthopedic surgeon with
At the frontier of tissue engineering is usage of human embryonic stem cells instead of adult bone marrow cells. Elisseeff demonstrated that when combined with appropriate biomaterials, these cells can be transformed into bone or cartilage tissue. Although promising, clinical applications of stem cell technologies are hampered by safety and cost issues. “We don’t want to invent something that is so expensive that it can’t be used. We need something that’s broadly applicable and usually works. If you can use in situ cells, then you have safety and good economics,” concludes Marcus. New technologies and materials for adhesion and integration to the cartilage are expected to improve orthopedic and other surgical interventions, including repairing disks in the back and sealing cataract incisions in the eye. This work is supported in part by the National Institute of Biomedical Imaging and Bioengineering.Reference: Wang D-A, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 6(5):385-92; 2007.
|
 |
 |
Department of Health and Human Services |
 |
National Institutes of Health |
 |