Home > Health & Education > eAdvances 
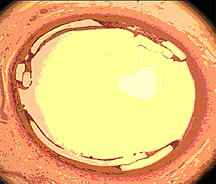
The Amazing Disappearing Stent: September 21, 2004Each year more than 1 million patients with coronary artery disease are treated with stents, according to the National Center for Health Statistics. These small wire-mesh tubes, usually made of stainless steel or another metal, are inserted into the small arteries surrounding the heart to keep them open. In some cases, however, the stented artery narrows again. This process, called restenosis, may occur because the rigid metal stent injures the artery walls and causes cells there to proliferate. To treat coronary artery disease and address the problem of restenosis, scientists have developed a stent made from a polymer that is less rigid than metal and that slowly degrades into harmless byproducts. Early tests in pig coronary arteries indicate that the stent keeps arteries open as effectively as metal stents, while causing negligible inflammation and blood clot formation. Bio-Compatible PolymersDesigning a new type of stent is challenging because stents must be both tough and flexible. They must be tough enough to withstand being secured tightly around a balloon catheter, yet be small and flexible enough to be threaded through winding blood vessels before reaching their destination in the coronary arteries, which supply blood to the heart. When X-rays show that a metal stent has entered the narrowed section of an artery, a surgeon expands the balloon, which bends and deforms the metal of the stent outward until it presses firmly against the inner wall of the artery. The stent then stays in place for the rest of a patient's life. When researchers at REVA Medical, Inc., in San Diego first decided to create a degradable stent, they already had the design in hand. They had recently developed a metal stent with a novel "slide and lock" design, in which the stent unfolds in the artery like an extension ladder instead of bending and expanding outward like most other stents. To find the right material for the degradable stent, REVA Medical turned to Dr. Joachim Kohn, the Board of Governors Professor of Chemistry at Rutgers University in New Jersey. Dr. Kohn specializes in developing biomaterials from natural substances found within the body. "The company gave me a set of requirements that the biomaterial had to fulfill," says Dr. Kohn. The material that came closest to matching the requirements was poly(DTE carbonate), a polymer that Dr. Kohn had synthesized in 1990. A polymer is a long molecule consisting of repeating chemical units called monomers. In this case, the monomer unit was derived from L-tyrosine, a common nutrient. Poly(DTE carbonate) was strong and nontoxic, but it had a few shortcomings: It took a long time to degrade, was not visible in X-ray images, and it was unknown whether it would provoke blood clot formation within arteries. Funded in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), Dr. Kohn and his colleagues set out to enhance poly(DTE carbonate) for use in a degradable stent. The Right IngredientsDr. Kohn reasoned that the polymer might be improved by incorporating iodine (for X-ray visibility), poly(ethylene glycol) (to make it less adhesive to blood components), and an acidic co-monomer (for fine-tuning biodegradation). The key challenge was to identify the optimal amounts of each of these ingredients without having to synthesize and characterize large numbers of test materials. "The problem was that there was no way to predict how changes in one ingredient, such as iodine, might affect the overall polymer performance relative to changes in any of the other ingredients," says Dr. Kohn. "Taking the traditional materials design approach would have involved sequentially synthesizing hundreds of different materials, each with slightly varying quantities of the added ingredients, and then testing each material until an acceptable polymer was found. Overall, this approach might have taken years." To address this challenge, Dr. Kohn developed a new design approach, which involved synthesizing a sample of potential polymer candidates, rapidly screening them for relevant properties, and then applying computer models to identify the relationships between polymer composition and material properties. The success of this approach depended, in large part, on the close collaboration between Dr. Kohn's laboratory at Rutgers University and scientists and engineers at REVA Medical. To identify polymers that would not induce blood clot formation within arteries, Dr. Kohn developed a new screening assay that measured the extent to which a material attracted fibrinogen, a blood protein that plays a key role in blood coagulation. In order to minimize blood clot formation, the material had to interact with fibrinogen as little as possible. With the new design approach, the scientists synthesized what they considered to be the ideal material candidate and then used it to produce a fully deployable stent, all within a record-breaking six months. In studies of the stents implanted for up to 28 days in the coronary arteries of live pigs, REVA Medical showed that the stents appeared to be biocompatible, producing negligible inflammation and blood clot formation. When Dr. Kohn developed a version of the material that was opaque to X-rays, REVA Medical demonstrated that X-ray images of the implanted stents were just as visible as a type of metal stent often used in patients. "One of the main benefits of our polymer stent is that it degrades over time, which provides more retreatment opportunities for the patient if there is restenosis," says Dr. Joan Zeltinger, Vice President of Scientific Affairs at REVA Medical. Dr. Kohn's research on the development of the new approach to biomaterials design is supported by NIBIB, REVA Medical, Inc., and the New Jersey Center for Biomaterials. References: Zeltinger J, Schmid E, Brandom D, Bolikal D, Pesnell A, Kohn J. Advances in the development of coronary stents. Biomaterials Forum 26:8-9, 2004. Smith JR, Seyda A, Weber N, Knight D, Abramson S, Kohn J. Integration of combinatorial synthesis, rapid screening, and computational modeling in biomaterials development. Macromolecular Rapid Communications 25:127-140, 2004. |
 |
 |
Department of Health and Human Services |
 |
National Institutes of Health |
 |